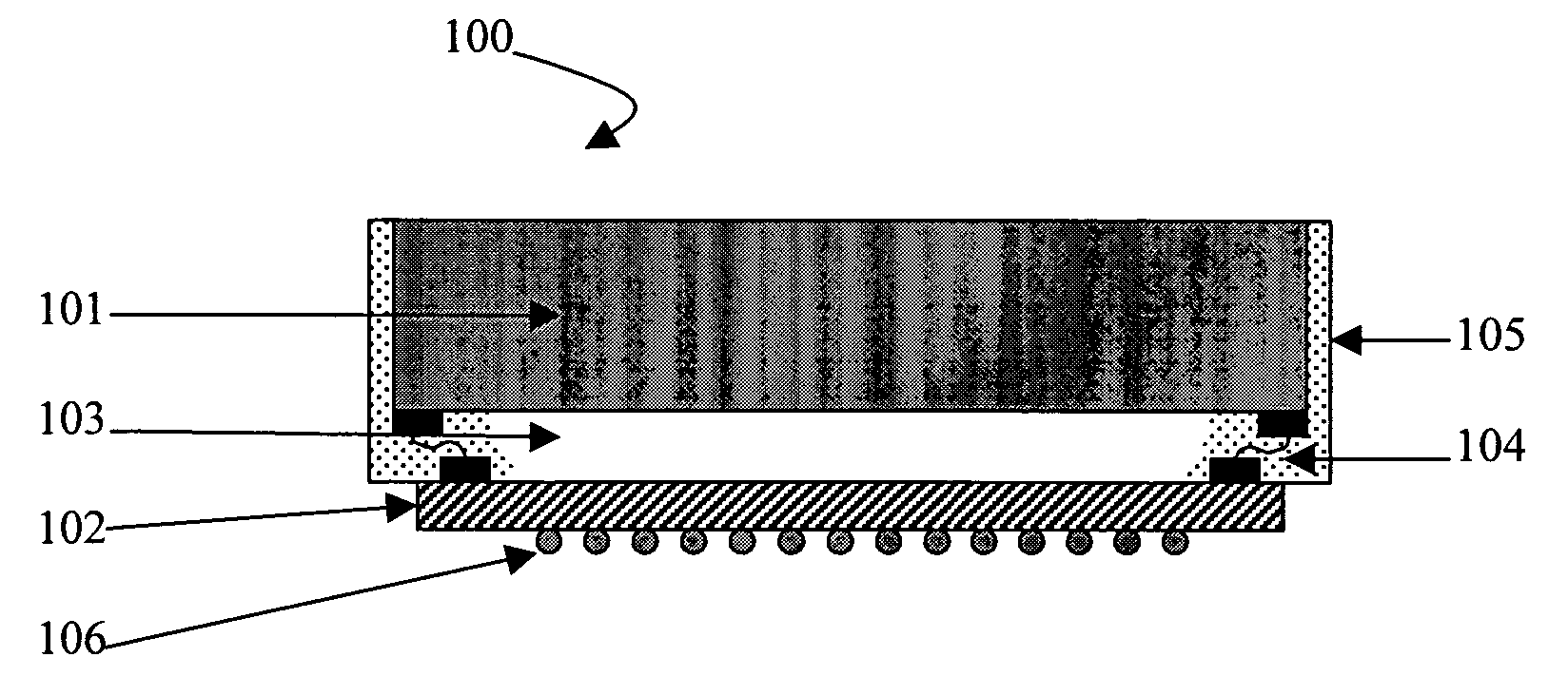
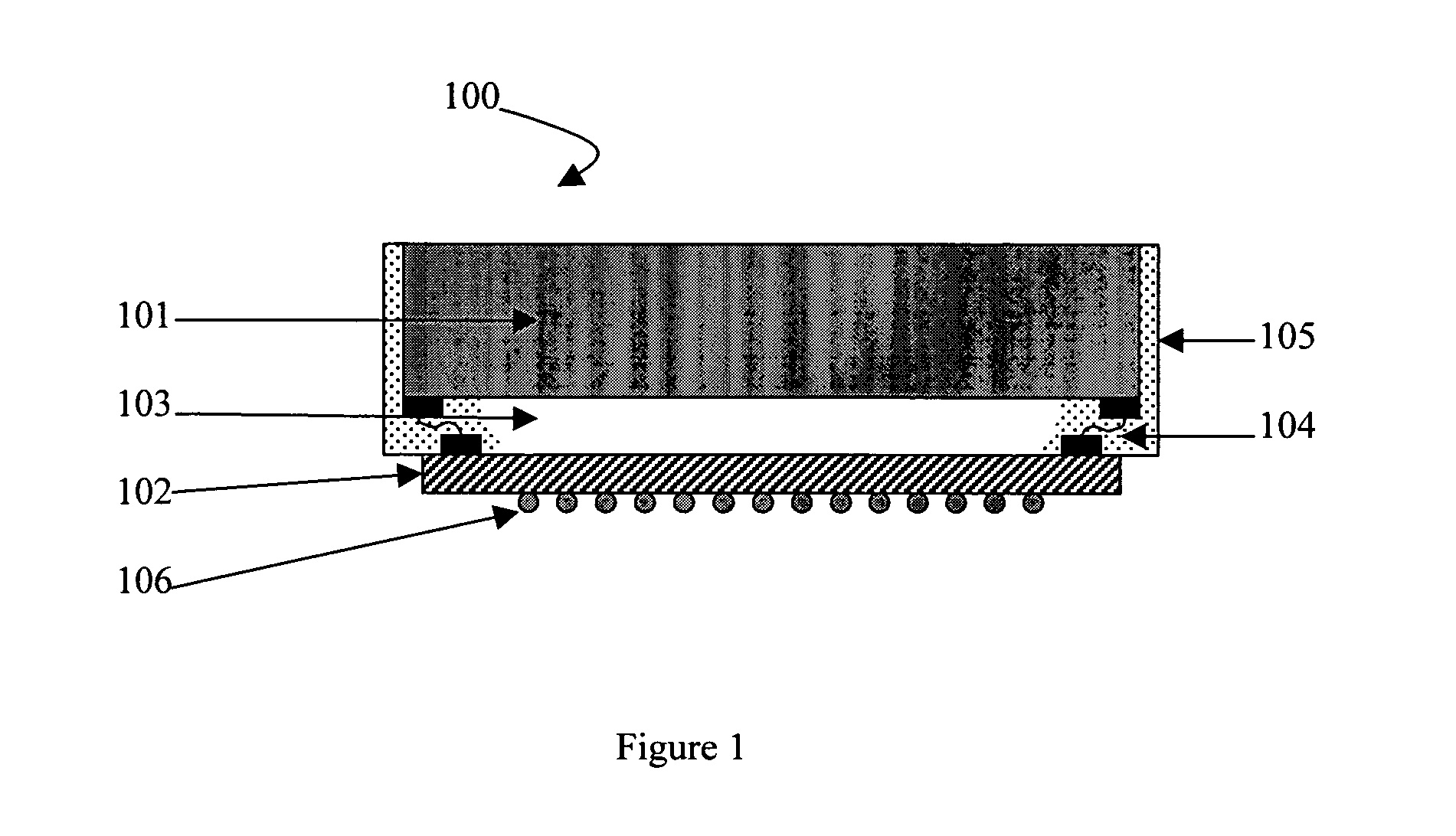
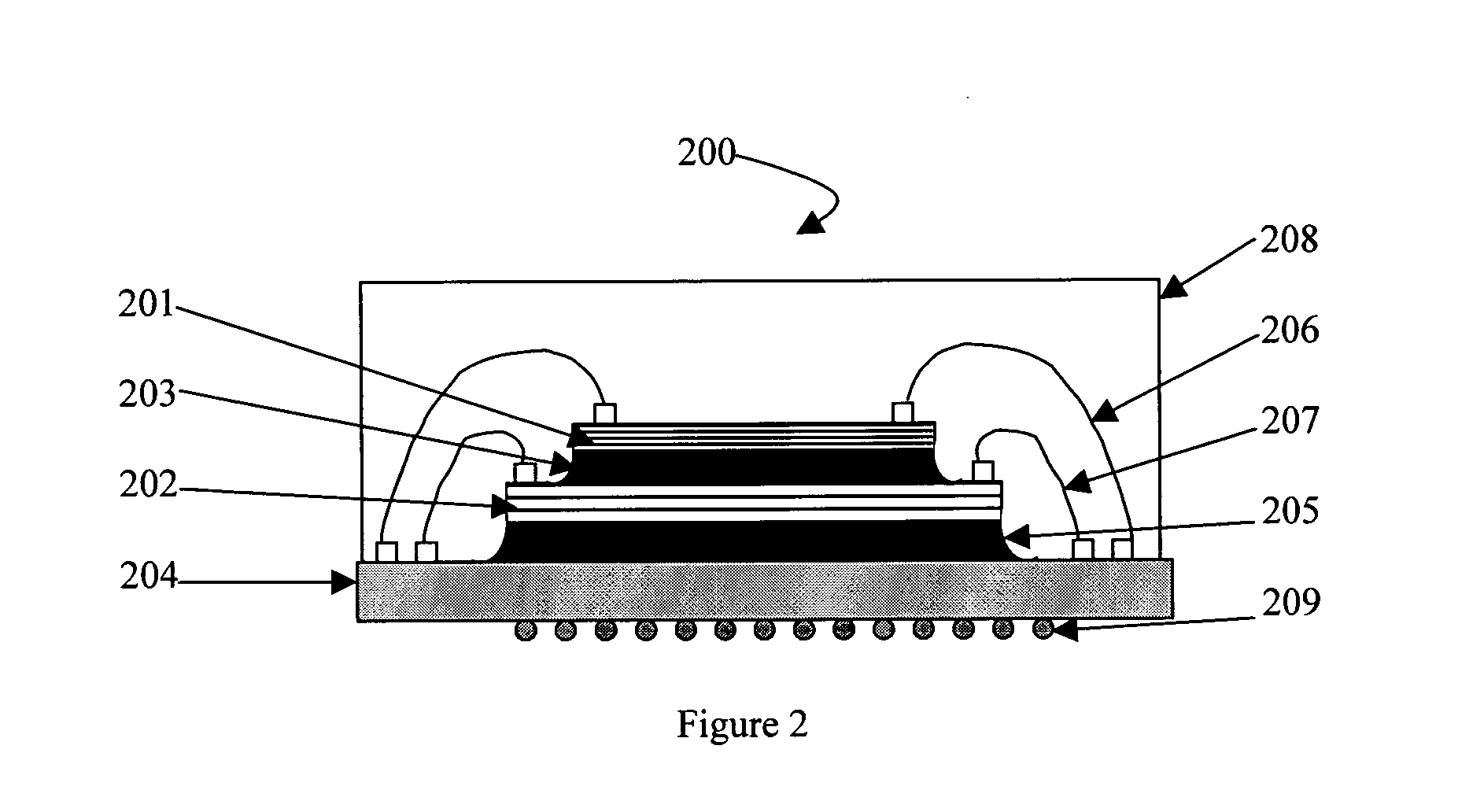
Silicones having improved chemical resistance and curable silicone compositions having improved migration resistance
a technology of curable silicone and composition, applied in the field of curable silicone composition and silicone, can solve the problems of high cost of fluorosilicone elastomers, solvents and engine oils, and poor resistance of polyorganosiloxane elastomers to some organic chemicals, and achieve the effect of improving resistance to bleed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
Sample Preparation and Analysis
A base is prepared by milling the following components in a 1 quart Ross mixer: Filler 1, Filler 2, Pigment, and the Polymer or Polymers selected for each example.
A blend is prepared by blending Cure Modifier and Organohydrogenpolysiloxane or Fluoroorganosilicone, or both, and heating for at least 30 minutes at 70 ° C.
The base, the blend, and any additional Polymer or Polymers are combined in a 20 gram dental mixer cup and are mixed at 3,500 revolutions per minute for 30 seconds. The resulting mixture is allowed to cool and Catalyst is added and is hand mixed for 10 seconds. The resulting mixture is again mixed at 3,500 revolutions per minute for 10 seconds with the dental mixer.
Rate of Bleed is measured as follows. A sample is allowed to equilibrate to room temperature. A 9 mm (length) piece of Continuous Au / Cu PI TAB tape purchased from Hitachi Cable is cut and taped to the middle section of a stainless steel print frame that fits in a vacuu...
examples 6-8
Compositions Containing Component (II)
Samples are prepared according to the method of Reference Example 1. The components and amounts for examples 6-8 are in Table 3. Extent of Bleed is measured as % change after 24 hours. The results are in Table 3.
TABLE 3Example #678Polymer 133.2632.9632.83Adhesion Promoter0.80.80.8Pigment0.40.40.4Filler 1111Filler 2606060Organohydrogenpolysiloxane0.80.40.21Fluoroorganosilicone 12.423.123.45Cure Modifier0.050.050.05Catalyst 11.271.271.27Extent of Bleed13.2%17.8%16.6%SiHtot / Vitot1.501.501.50
As shown in the examples and comparative examples above, compositions containing adhesion promoters but no fluoroorganosilicones exhibit greater Bleed than the compositions that contain neither adhesion promoters nor fluoroorganosilicones and the compositions that contain fluoroorganosilicones but no adhesion promoters. However, it was surprisingly found that the compositions containing both adhesion promoters and fluoroorganosilicones exhibit less Bleed t...
reference example 2
Sample Preparation and Analysis
Samples are prepared by combining Polymer 2, Pigment, Spacer, filler 2, Crosslinker 1, Adhesion Promoter, Catalyst 2, Adhesion Promoter, and any Phenylsiloxanes and Crosslinkers.
Bleed is measured as the percent increase in diameter of a dot of sample placed on an electronic circuit tape. The circuit tape is Hitachi polyimide TAB Tape LC-TAB μBGA 220A14, which consists of gold plated copper circuit traces with Tomoegawa X epoxy laminate adhesive between the traces. Dots of sample with diameters ranging from 0.914 mm to 1.953 mm are placed on to the epoxy surface on this circuit tape. Immediately after the dots are applied to the epoxy surface, pictures of the dots are taken and the time of placement is recorded along with the picture.
As time passes, subsequent pictures of the dots are taken using identical magnifications. All pictures are taken with a Nikon sMZ-10A microscope, a Diagnostic Instruments, Inc. Spot Jr Model 1.5.0 digital camera, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com