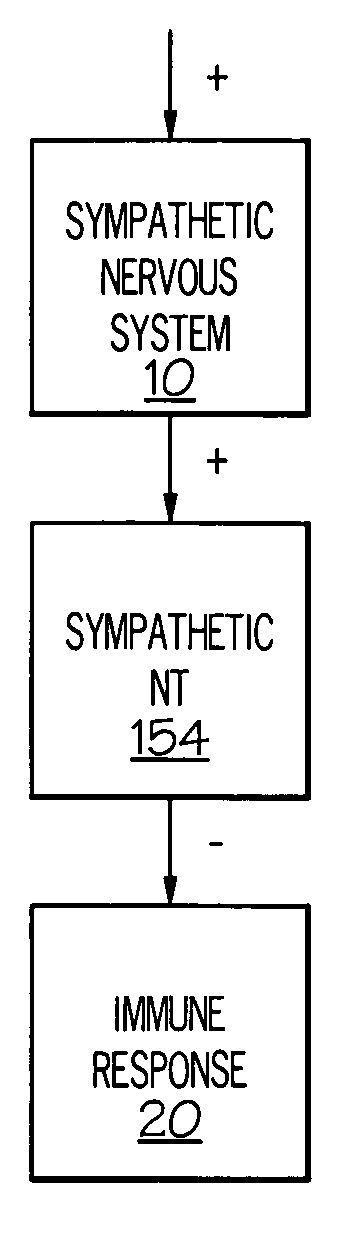
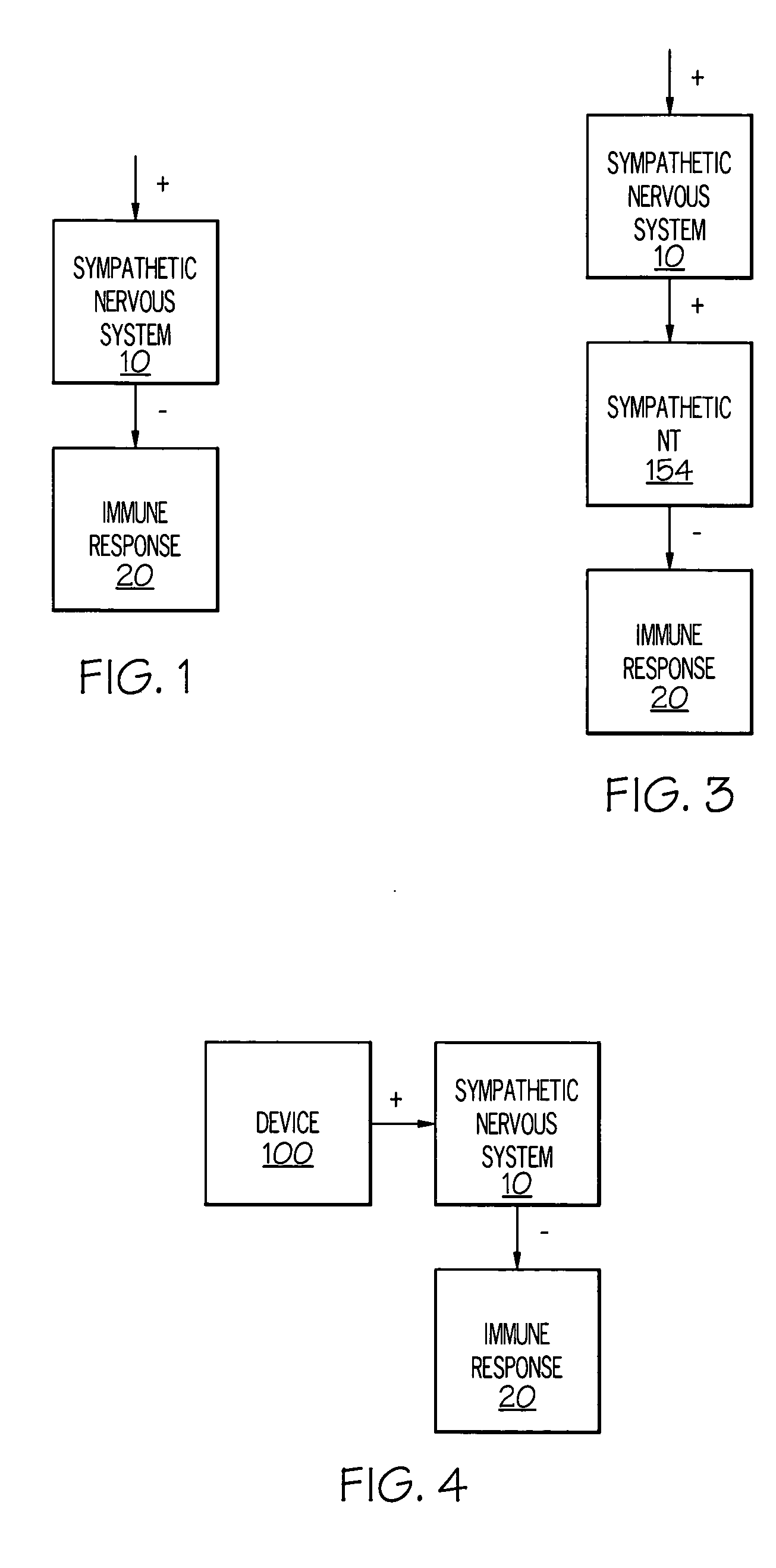
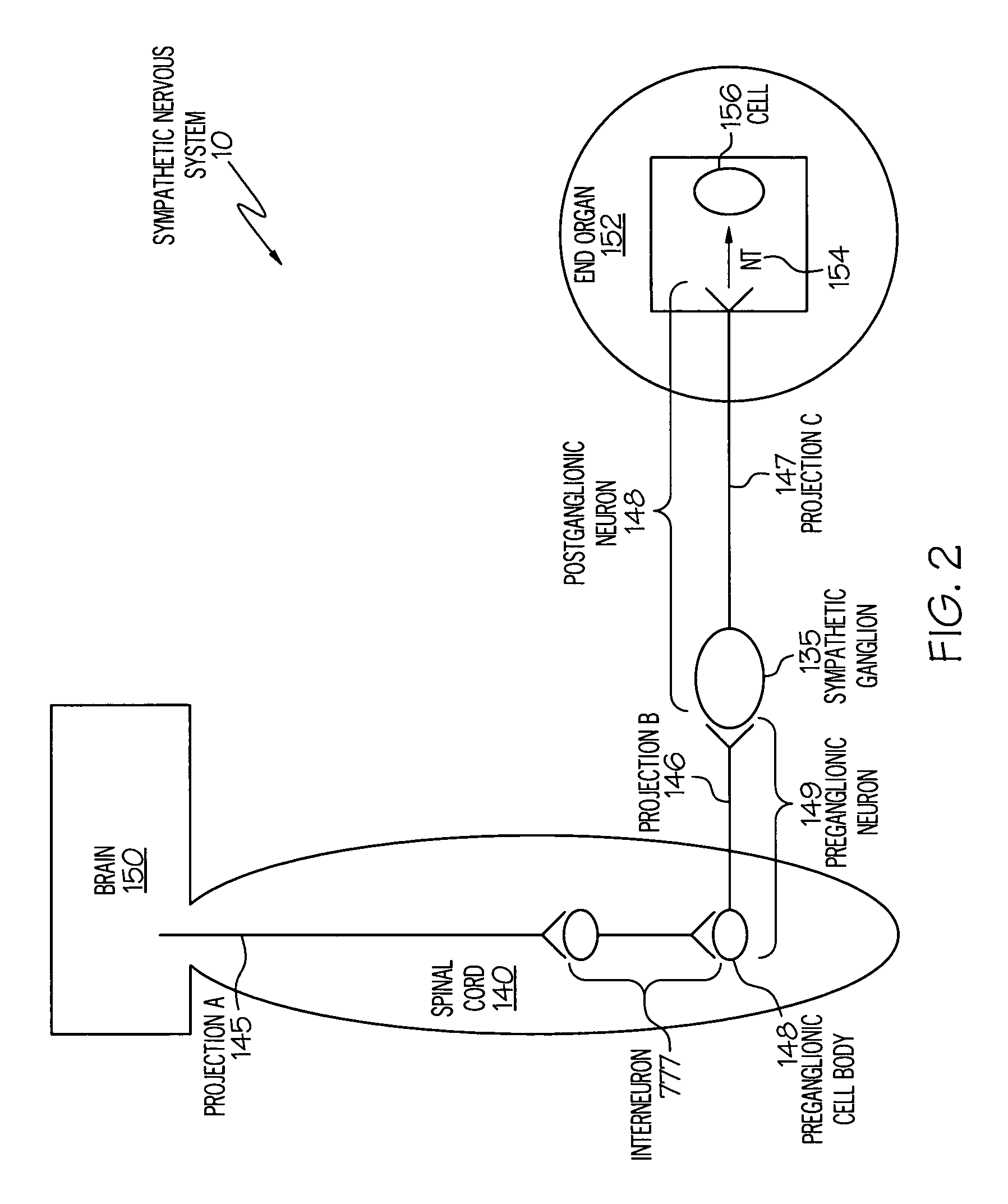
Device and method for inhibiting release of pro-inflammatory mediator
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0127] The following example is provided to illustrate specific embodiments of the invention only, and should not be construed as limiting the scope of the invention.
[0128] Methods
[0129] A porcine model of endotoxic shock was established previously for use in the following procedure. Here, the porcine model was validated and the effects of systemic LPS administration (decreased WBC count, increased pro-inflammatory cytokines, etc.) were demonstrated to be consistent with sepsis models of other species. The dose of endotoxin administered is generally lethal within 8 hrs if no attempt at recovery is made. Pigs (50-80 kg) were housed at 22° C. on a 12 hr light / dark cycle. The animals were cared for and housed at Physiological Research Laboratories (Coon Rapids, Minn.) in individual runs that meet the weight-space specifications recommended in The Guide for the Care and Use of Laboratory Animals. The protocol was in compliance with the Animal Welfare Act of 1966 (P. L. 89-544), and al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com