Ca-Mg-Ni containing alloys, method for preparing the same and use thereof for gas phase hydrogen storage
a technology of ni-containing alloys and gas phase hydrogen storage, which is applied in the direction of fuel cell auxiliaries, inorganic chemistry, fuel cells, etc., can solve the problems of low gravimetric storage density, high cost, and inability to widen the use of hydrogen energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-1
Compound According to the Prior Art Made by the Method According to the Invention
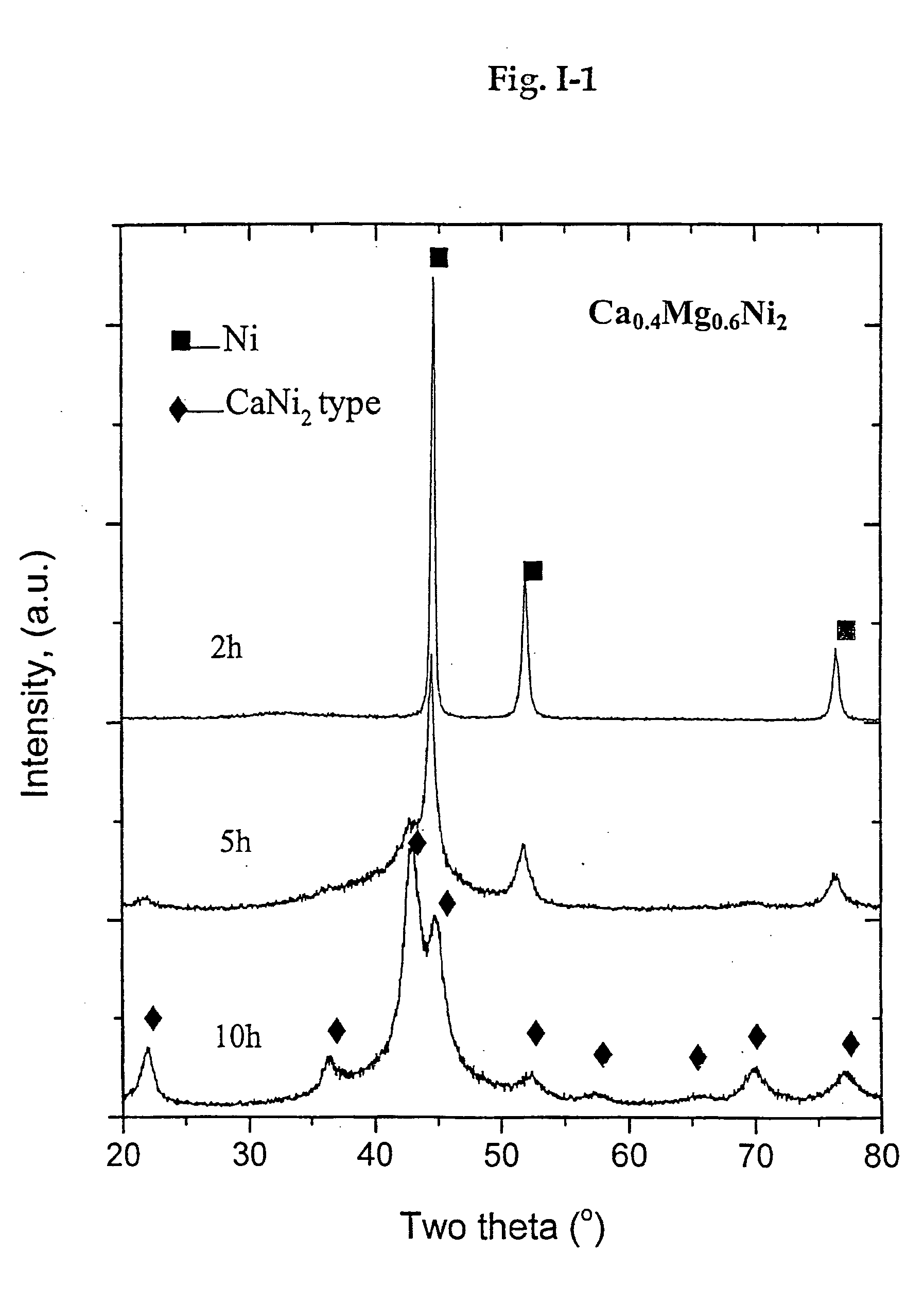
[0128] Ca0.3Mg0.7Ni2 (b=1, x=0.1, y=−0.1, z=0) was synthesized in a SPEX® high energy ball mill under the protection of argon. A Mg powder (>99%, +100 mesh), Ca granules (>99.5, ˜2 mm in size) and a Ni powder (<99.9%, −325 mesh) were used as starting materials.
[0129] Isothermal annealing was performed in a tubular furnace under the protection of argon. The mechanically alloyed powder was sealed in a stainless steel crucible before annealing. The powder was heated to 1000° C. at a heating rate of 30 C / min, and held at 1000° C. for 1 hour, then cooled down to room temperature in the furnace.
[0130] Hydrogen absorption / desorption properties were measured by using an automated Sievert's type apparatus. The annealed powder normally needs mild activation treatment, such as heated to 200° C. under vacuum and then cooled down. EDX analysis shows that the Fe content in the end product less than 0.2 at. %. The ...
example 1-2
Compound According to the Invention Made by the Method According to the Invention
[0131] Ca0.15Mg0.7Mm0.15Ni2 (x=0.25, y=−0.1, M=Mm, x+y=0.15, b=1, z=0) was synthesized by mechanical alloying of elemental powder blends. The alloy was annealed in the same manner as in Example 1-1. This alloy had a hydrogen storage capacity of 1.25 wt. %. The plateau pressure was drastically raised, and the plateau slope was much less than that of Ca0.3 Mg0.7Ni2 of the Example 1-1
examples 1-3
AND SUBSEQUENT
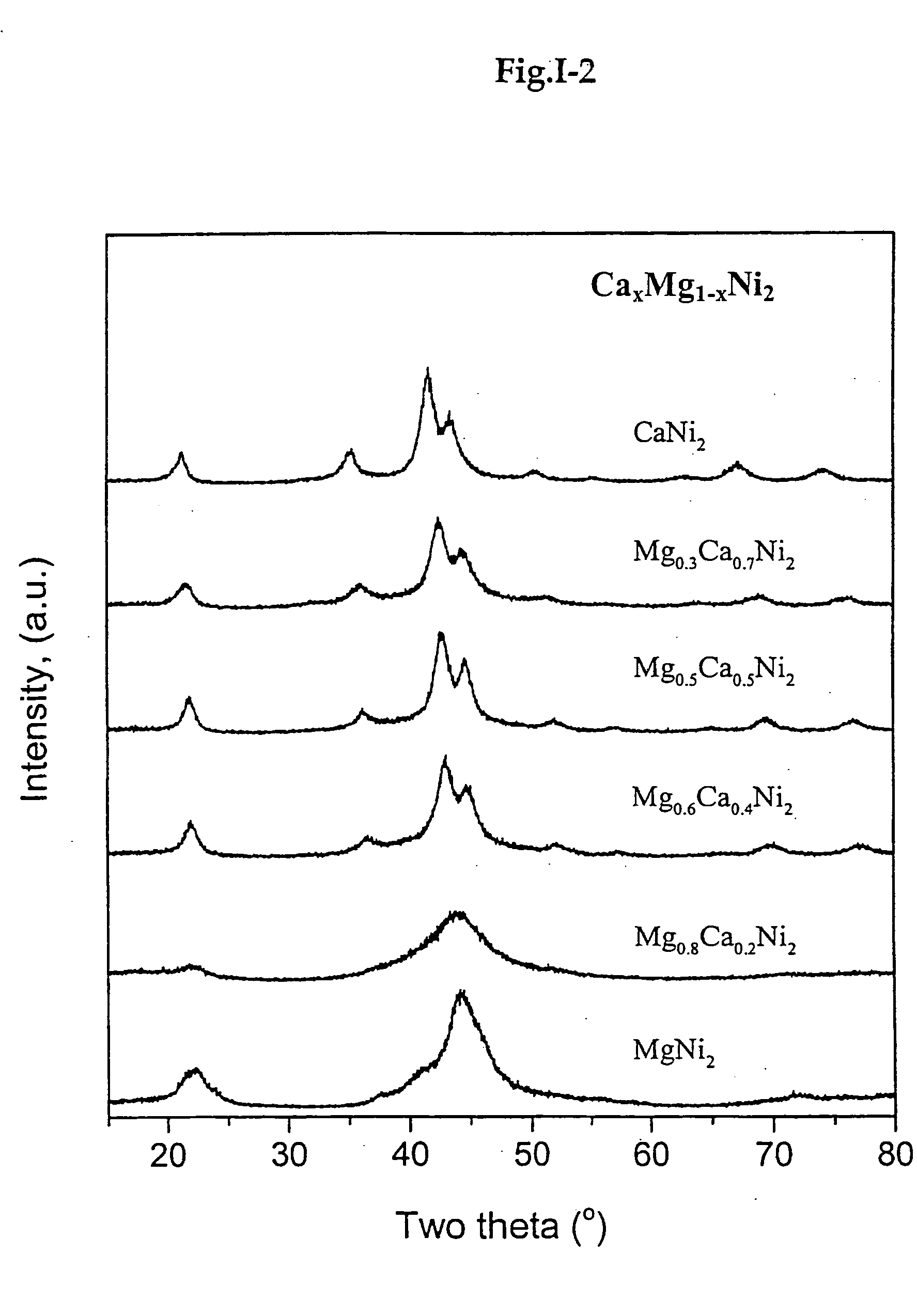
[0132] Other examples similar to Example 1-2 were carried out. The results of these other examples are reported in the accompanying drawings (see FIGS. 1-1 to 1-9).
2. Alloys of the A2B5 Type
[0133] In another aspect, the present invention provides an A2B5 type alloy according to the formula:
(Ca0.4-xMg0.6-yMx+y)b(Ni1-zTz)5
where [0134] M is at least one metal selected from the group consisting of Y, Ce, La, Pr, Nd, Th, Nd, Ti, V, Zr, Ta, Hf, Sr, Ba and mischmetals; [0135] T is at least one element selected from the group consisting of Al, Zn, Cu, Fe, Co, Mn, Cr, Mo, W, Si, Ga, Ge, In, Sn, Ag, C and B; [0136] 1.75≦b≦2.25, [0137]−0.4≦x≦0.2, [0138]−0.2≦y≦0.4, [0139] x+y≧0, and [0140] 0≦z≦0.5.
[0141] Preferably: [0142]−0.1≦x≦0.1, and [0143]−0.1≦y≦0.2.
[0144] Preferably also, Mg and Ca are present in a Mg / Ca ratio ranging from 0.5 to 2 and more preferably from 1.5 to 1.75.
(a) Ca—Mg—Ni Alloys of the A2B5 Type
[0145] As mentioned before, in a Ca—Ni system, there are four...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com