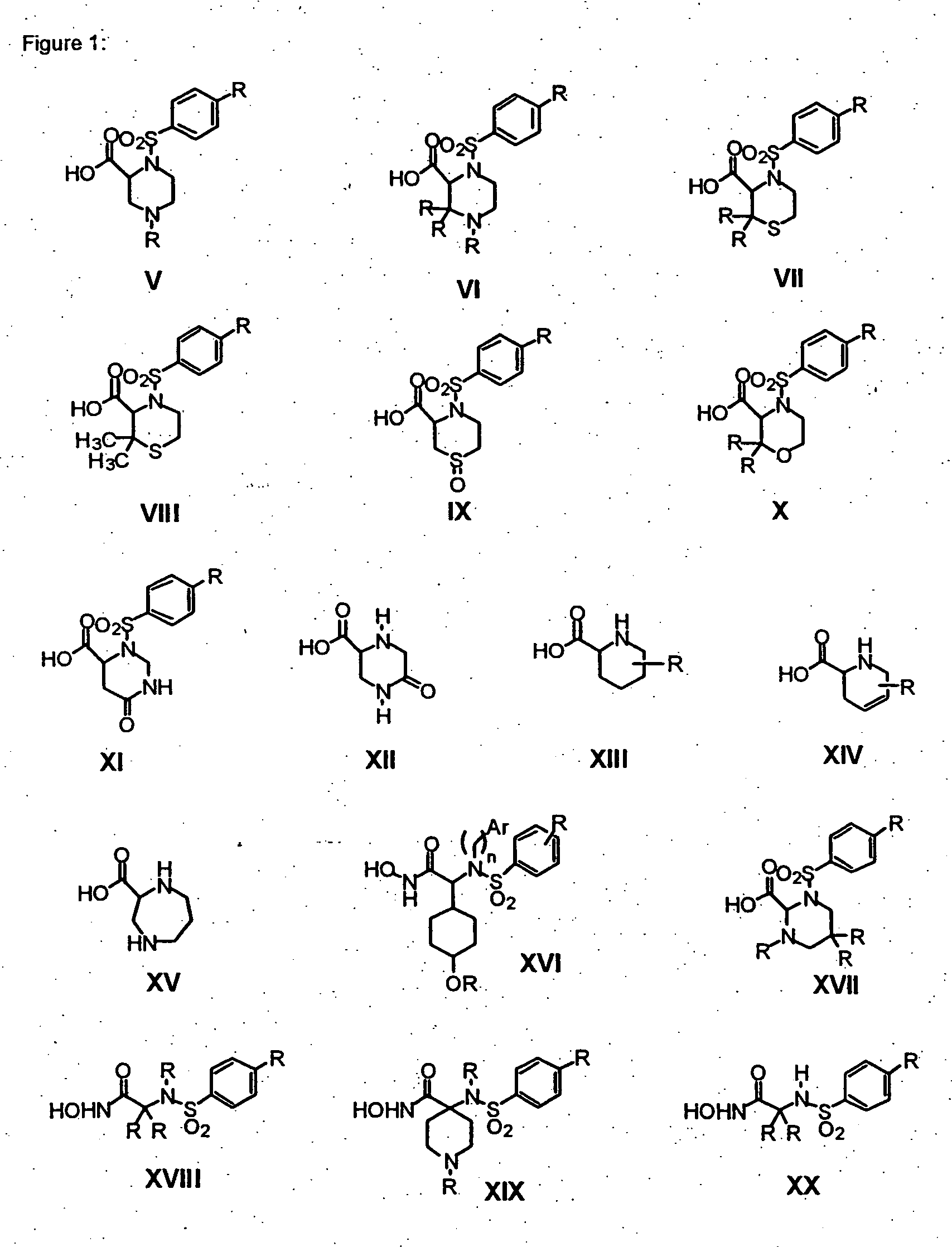
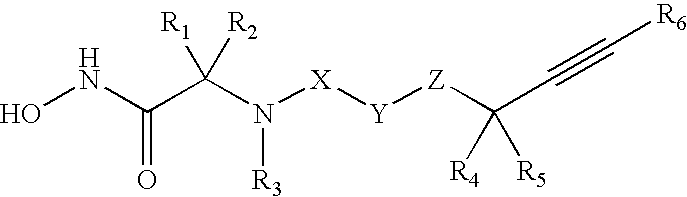
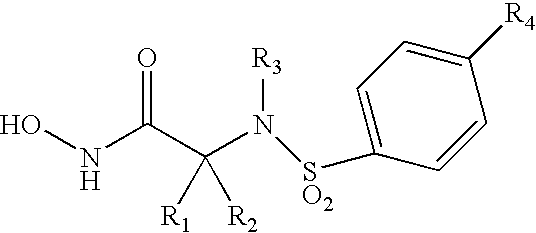
Acetylenic alpha-amino acid-based sulfonamide hydroxamic acid tace inhibitors
a technology of acetyl aryl sulfonamide and tace inhibitor, which is applied in the direction of phosphorous compound active ingredients, biocide, group 5/15 element organic compounds, etc., can solve the problems of bioavailability and pharmacokinetic problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
4-But-2-ynyloxy-benzenesulfonic Acid Sodium Salt
[0452] To a solution of 52.35 g (0.225 mol) of 4-hydroxybenzenesulfonate sodium salt in 1 L of isopropanol and 225 mL of a 1.0N solution of sodium hydroxide was added 59.96 g (0.45 mol) of 1-bromo-2-butyne. The resulting mixture was heated to 70° for 15 h and then the isopropanol was removed by evaporation in vacuo. The resulting white precipitate was collected by filtration, washed with isopropanol and ether and dried in vacuo to give 56.0 g (100%) of the butynyl ether as a white solid.
example 2
4-But-2-ynyloxy-benzenesulfonyl Chloride
[0453] To a 0° solution of 43.8 mL (0.087 mol) of 2M oxalyl chloride / dichloroethane solution in 29 mL of dichloromethane was dropwise added 6.77 mL (0.087 mol) of DMF followed by 7.24 g (0.029 mol) of the product of Example 1. The reaction mixture was stirred for 10 minutes at 0° then let warm to room temperature and stirred for 2 days. The reaction was then poured into ice and extracted with 150 mL of hexanes. The organics were washed with water and brine, dried over Na2SO4, filtered and concentrated in vacuo to provide 6.23 g (88%) of the sulfonyl chloride as a yellow solid; m.p. 63-65° C. EI Mass Spec: 243.9 (M+).
example 3
But-2-ynyloxy-benzene
[0454] To a solution of 6.14 g (0.023 mol) of triphenylphosphine dissolved in 100 mL of benzene and 40 mL of THF was added 1.75 mL (0.023 mol) of 2-butyn-1-ol. After five minutes 2.00 (0.023 mol) phenol, dissolved in 10 mL of THF, was added to the reaction followed by 3.69 mL (0.023 mol) of diethyl azodicarboxylate. The resulting reaction mixture was stirred for 18 h at room temperature and then concentrated in vacuo. The residue was chromatographed on silica gel eluting with ethyl acetate / hexanes (1:10) to provide 2.18 g (70%) of the butynyl ether as a clear liquid. EI Mass Spec: 146.0 MH+
PUM
| Property | Measurement | Unit |
|---|---|---|
| Polarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com