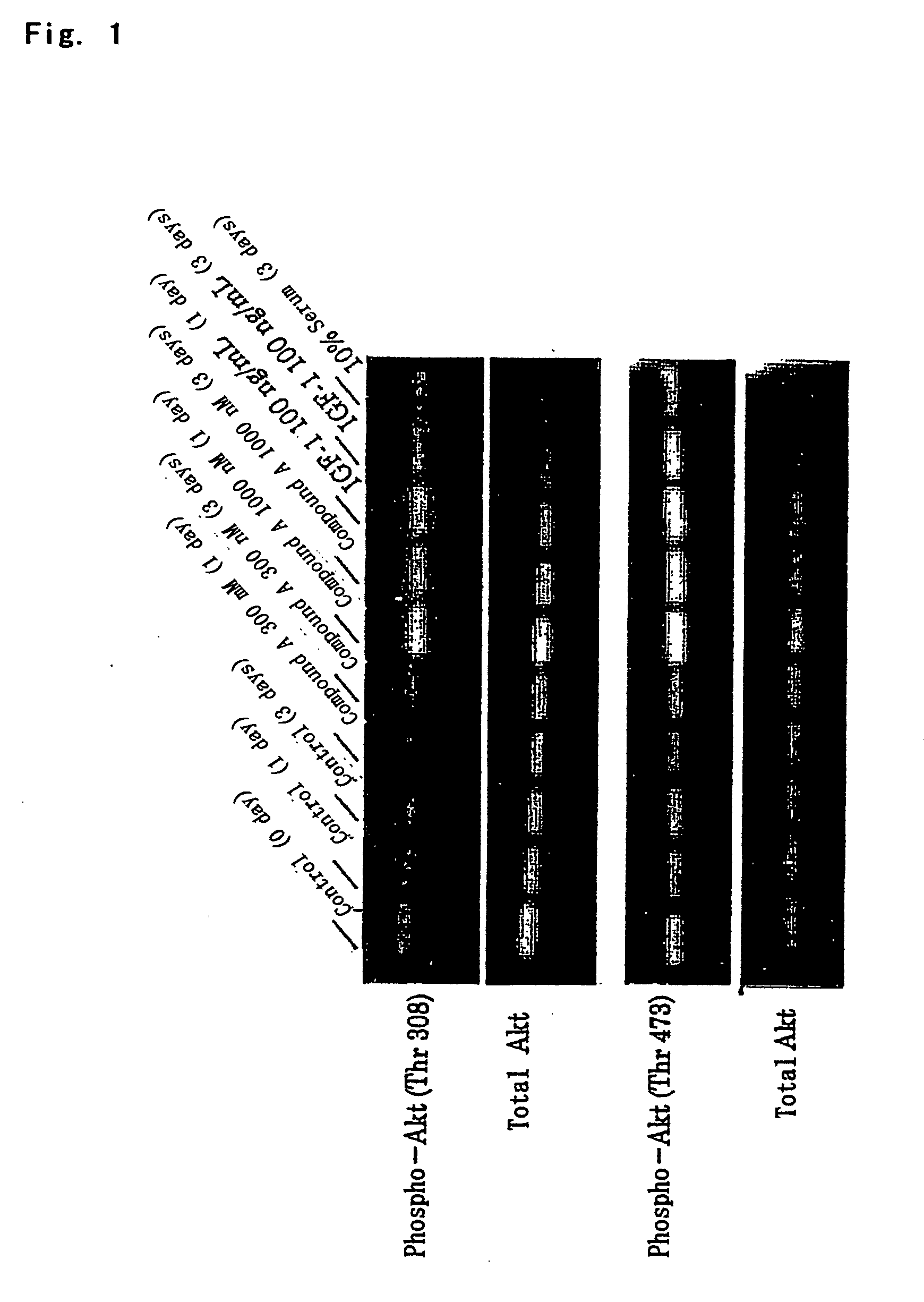
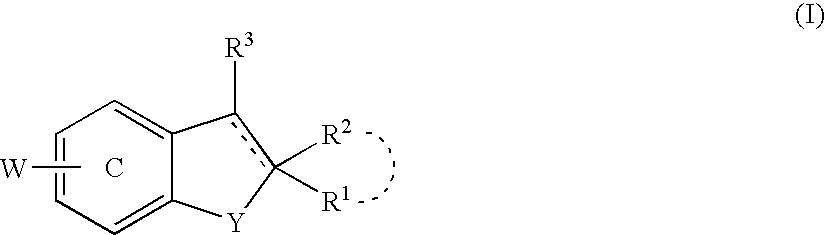
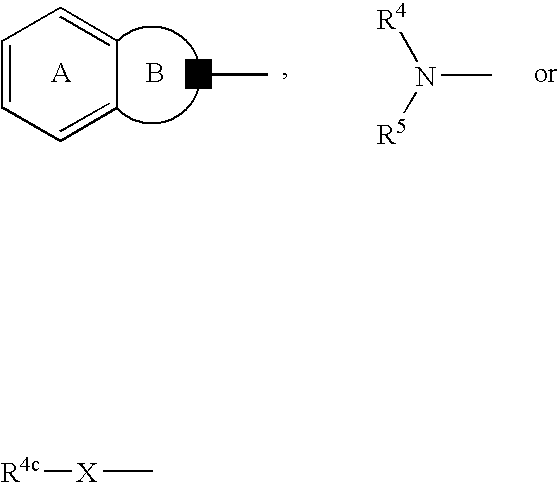
Antidepressant
a technology of antidepressant and protein kinase, which is applied in the field of protein kinase b, can solve the problems of insufficient regeneration of nerve endings, gradual loss of effects with disease progression, and inability to confirm the activation and effect of parkinson's disease in vitro, so as to promote nerve regeneration, maintain the survival of nerve cells, and improve the effect of therapeutic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1a
[0406]
(1)Compound 14a50mg(2)Lactose34mg(3)Corn starch10.6mg(4)Corn starch (paste)5mg(5)Magnesium stearate0.4mg(6)Carboxymethylcellulose calcium20mgTotal120mg
[0407] According to a conventional method, the above (1) to (6) are mixed and compressed into a tablet with a tableting machine.
preparation example 1b
[0408]
(1)Compound 19b50mg(2)Lactose34mg(3)Corn starch10.6mg(4)Corn starch (paste)5mg(5)Magnesium stearate0.4mg(6)Carboxymethylcellulose calcium20mgTotal120mg
[0409] According to a conventional method, the above (1) to (6) are mixed and compressed into a tablet with a tableting machine.
preparation example 1c
[0410]
(1)Compound 4c50mg(2)Lactose34mg(3)Corn starch10.6mg(4)Corn starch (paste)5mg(5)Magnesium stearate0.4mg(6)Carboxymethylcellulose calcium20mgTotal120mg
[0411] According to a conventional method, the above (1) to (6) were mixed and compressed into a tablet with a tableting machine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure | aaaaa | aaaaa |
| chemical structural formulas | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com