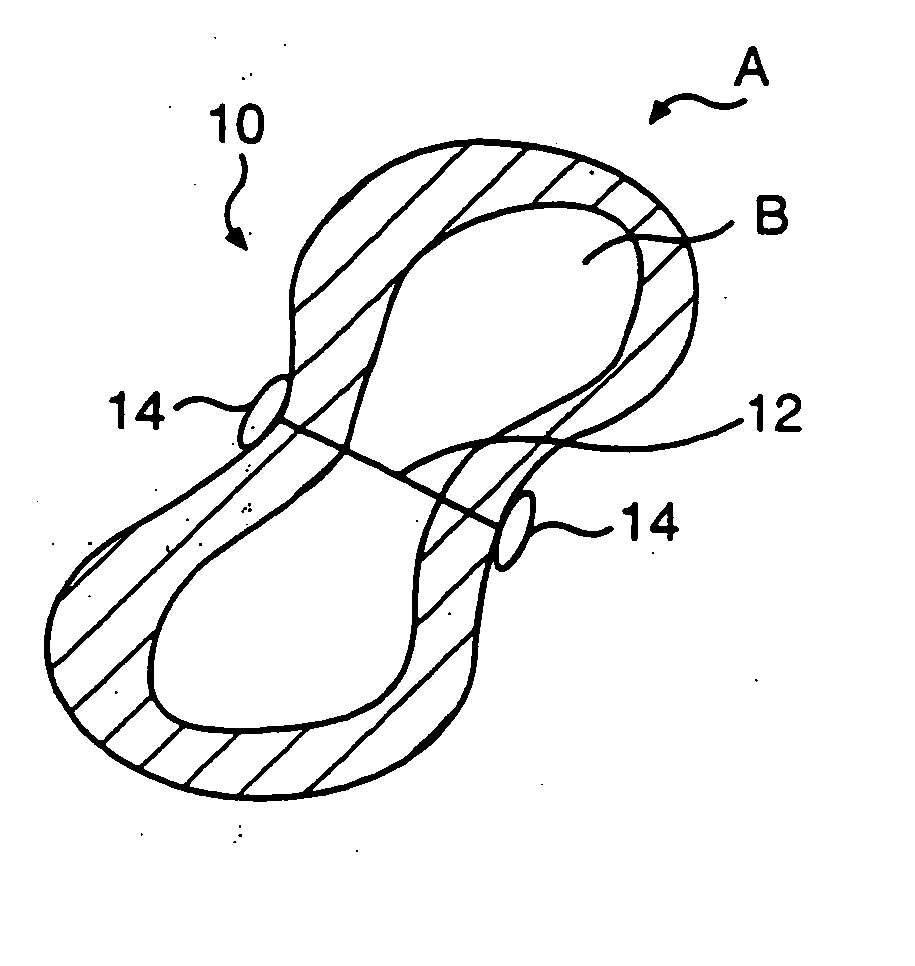
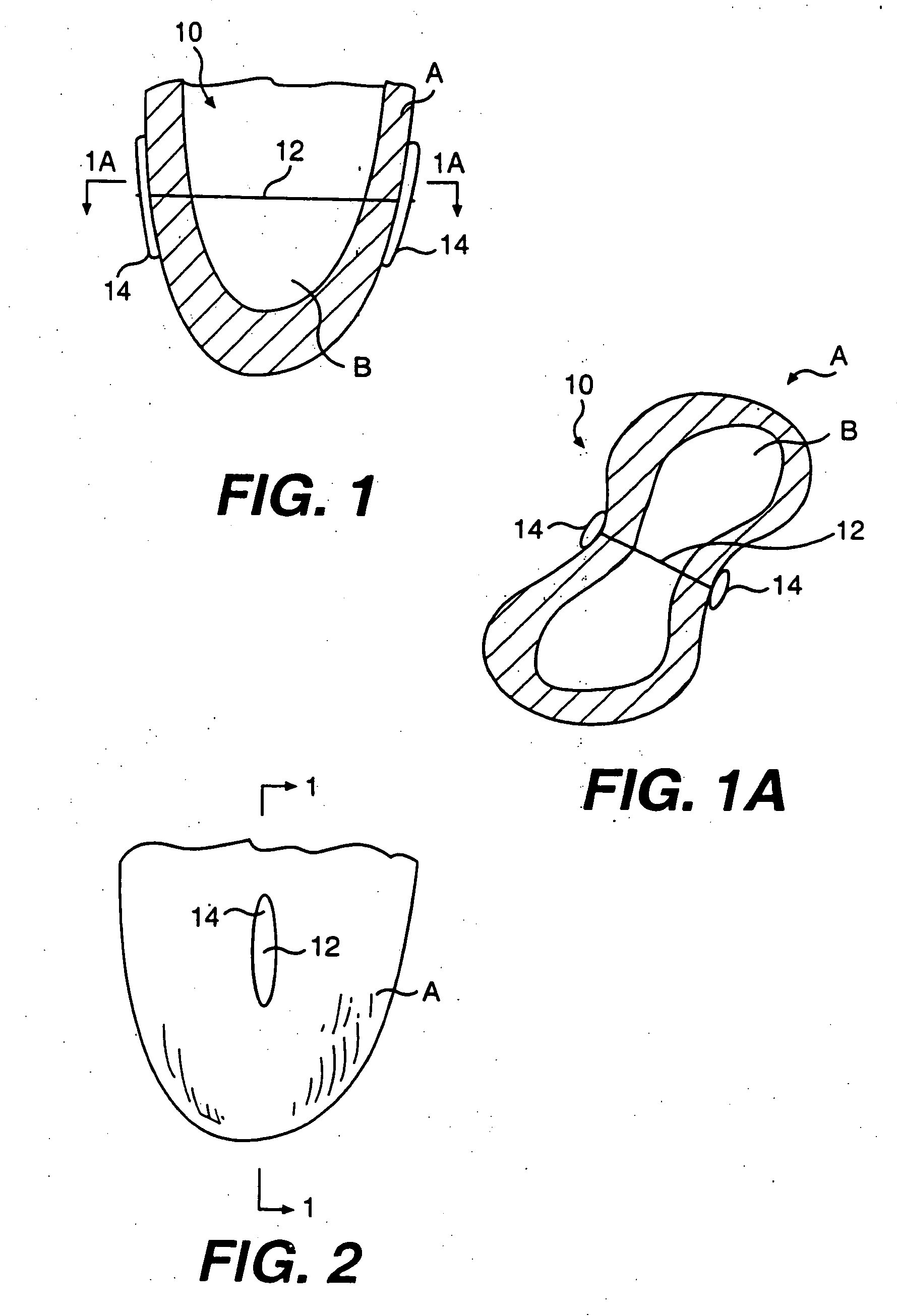
Transventricular implant tools and devices
a technology of transventricular implant and tool, which is applied in the field of applicative treatment of a failing heart, can solve the problems of increasing the requirement of systolic contraction of the wall tension, increasing the radius of curvature, and increasing the tension within the ventricle wall
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 141
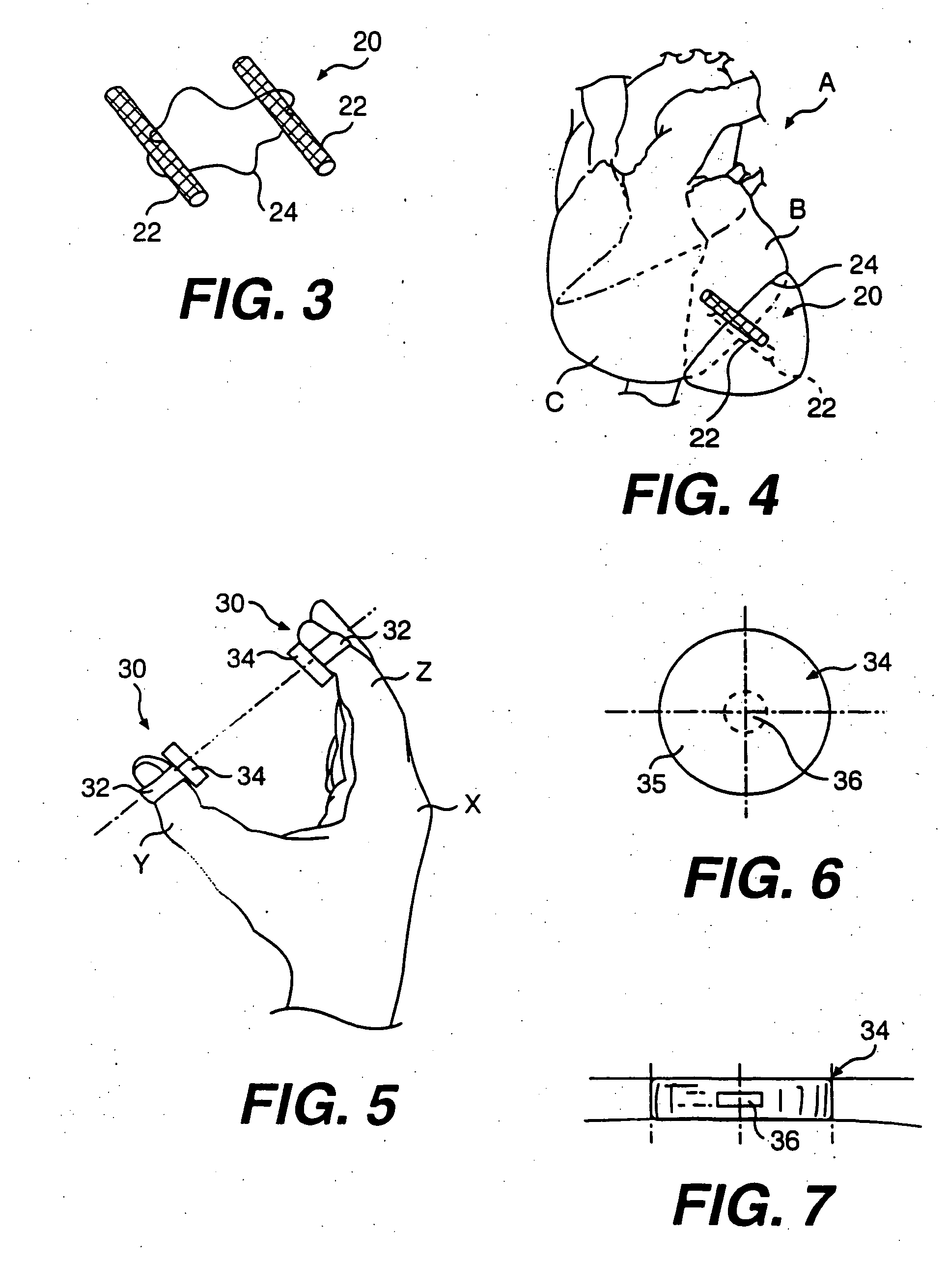
[0124]FIG. 19 is a perspective view of an alternate embodiment of an alignment device pad 141. Alternate embodiment 141 is disposed at the end of an alignment device arm 140. Pad 141 includes a funnel shape aperture 142. Aperture 142 includes a large diameter end 144 and a small diameter end 143. Large diameter end 144 is preferably disposed adjacent the heart and tension member exit point during use. A guide tube 145 can lead out from smaller diameter end 143 of aperture 142. Guide tube 145 preferably includes a bend passing through an arc of preferably between about 45° to about 135° and more preferably about 90°. The radius of the bend is preferably long enough that devices advanced through guide tube 145 are not permanently bent as a consequence of being advanced through the arc of guide tube 145. The radius of the arc is preferably about 0.05 inches to about 2 inches, and more preferably between about 0.75 inches and, most preferably about 1 inch as measured to the central axis...
embodiment 151
[0125]FIG. 20 is a perspective view of yet another alternate pad embodiment 151. Pad 151 has a similar shape to that of FIG. 18 and is disposed at the end of an alignment device arm 156. Pad 151 has an aperture 152 therethrough and a side notch 153 for transverse removal of a tension member guide and / or tension member. Extending from arm 156 is a stop arm 155 having a tension member guide stop 154 aligned with aperture 156 and spaced from pad 151. In use, stop 154 is disposed on the opposite side of pad 151 from the heart. As a tension member guide 157 is advanced from the heart through aperture 152, advancement of the tip of guide 157 is limited by needle stop 154. Stop 154 thus can limit additional advancement of guide 157 which might injure tissue adjacent to the heart.
[0126]FIG. 21 is a perspective view of an alignment device guide tube 165. Alignment device guide tube 165 preferably includes a luer lock or similar coupling 166 releasably connectable to a corresponding coupling ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com