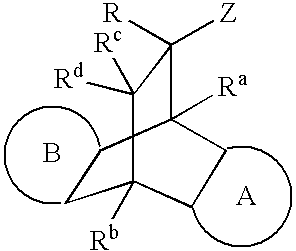
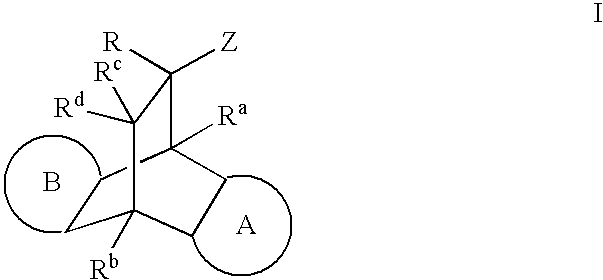
Modulators of the glucocorticoid receptor and method
a technology of glucocorticoid receptor and modulator, which is applied in the direction of drug composition, immunological disorders, metabolism disorders, etc., can solve the problem of limited systemic use of the receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation 1
2-Amino-4-[1-(4-fluoro)naphthyl]thiazole 1a
[0381]
Step 1
[0382] To a solution of 4′-fluoro-1′-acetonaphthone (28.69 mmol, 5.4 g) in 1,4-dioxane (18.0 mL) at 0° C. was added bromine (35.13 mmol, 5.61 g). After 3 hours at room temperature the reaction mixture was concentrated in vacuo to give 7.66 g (Y: δ 100%) of the product of step 1.
Step 2
[0383] To a solution of the product of step 1 (28.69 mmol, 7.66 g) in ethyl alcohol (20 mL) at room temperature was added thiourea (36.13 mmol, 2.75 g). After 1 hour at room temperature a precipitate formed. To the reaction mixture was added water (100 mL) and the solid was collected by vacuum filtration. The solid was then washed with water (3×100 mL) and dichloromethane (3×100 mL). The solid was then dried in vacuo to give 5.5 g (Y: 75%) of the title compound 1a. MS (E+) m / z: 245 (MH+).
[0384] In a similar manner the following compounds were prepared from the corresponding ketone.
PreparationStructure1b1c1d1e1f1g1h1i1j1k1l1m1n1o1p1q1r1s1t1u...
preparation 2
2-Amino-4-[1-(4-fluoro)naphthyl]imidazole 2a
[0385]
Step 1
[0386] To a solution of the product of preparation 1a, step 1 (18.73 mmol, 5.0 g) in DMF (15 mL) at room temperature was added 1-acetylguanidine (57.43 mmol, 5.80 g). After 5 hours at room temperature, the reaction mixture was diluted with water (100 mL) and extracted with ethyl acetate (3×100 mL). The organic phases were concentrated in vacuo and the residue chromatographed on silica gel (eluted with 5% methanol in dichloromethane) to give 2.0 g (Y: 39%) of the product of step 1. MS (E+) m / z: 270 (MH+).
Step 2
[0387] To a solution of the product of step 1 (7.43 mmol, 2.0 g) in methanol (17 mL) was added water (8.5 mL) and 12 N HCl (12.0 mL). After 1 hour at reflux the reaction mixture was concentrated in vacuo to approximately 15 mL. The resulting solution was then purified and neutralized by cation exchange SPE to give 1.66 g (Y: 99%) of the title compound 2a. MS (E+) m / z: 228 (MH+).
[0388] In a similar manner the followi...
preparation 3
2-Amino-4-(1-naphthyl)oxazole 3a
[0389]
Step 1
[0390] To a solution of 1-acetonaphthone (29.38 mmol, 5.0 g) in glacial acetic acid (10.0 mL) at RT was added bromine (30.06 mmol, 4.80 g) in glacial acetic acid (5.0 mL). After 5 minutes the reaction mixture was poured onto crushed ice and extracted with dichloromethane to give 7.31 g (Y: 100%) of the product of step 1. MS (E+) m / z: 250 (MH+).
Step 2
[0391] To a solution of the product of step 1 (5.50 mmol, 1.37 g) in ethyl alcohol (10 mL) was added urea (27.50 mmol, 1.65 g). After 2 hours at reflux the reaction mixture was concentrated in vacuo and the residue chromatographed on silica gel (eluted with 30% ethyl acetate in hexane) to give 100 mg (Y: 9%) of the title compound 3a. MS (E+) m / z: 211 (MH+).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Power | aaaaa | aaaaa |
| Power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com