Selective reduction of cupriferous calcine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Bessemer matte (Cu (40.7%), Ni (39.7%), Co (0.54%), Fe (0.91%) and S (18%)) was roasted in a 12 inch (30.4 cm) fluid bed. The calcine was used as feed for the reduction tests.
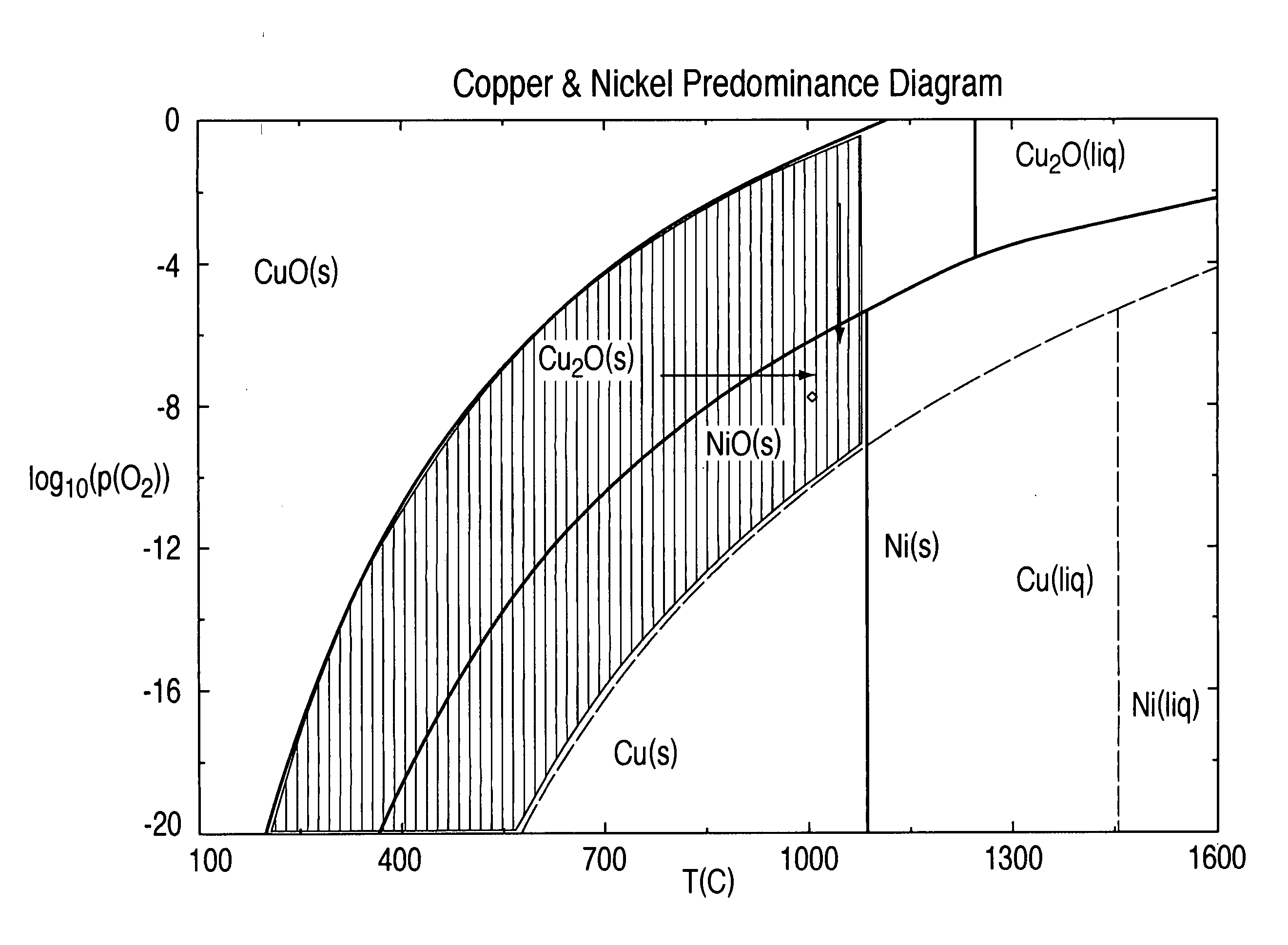
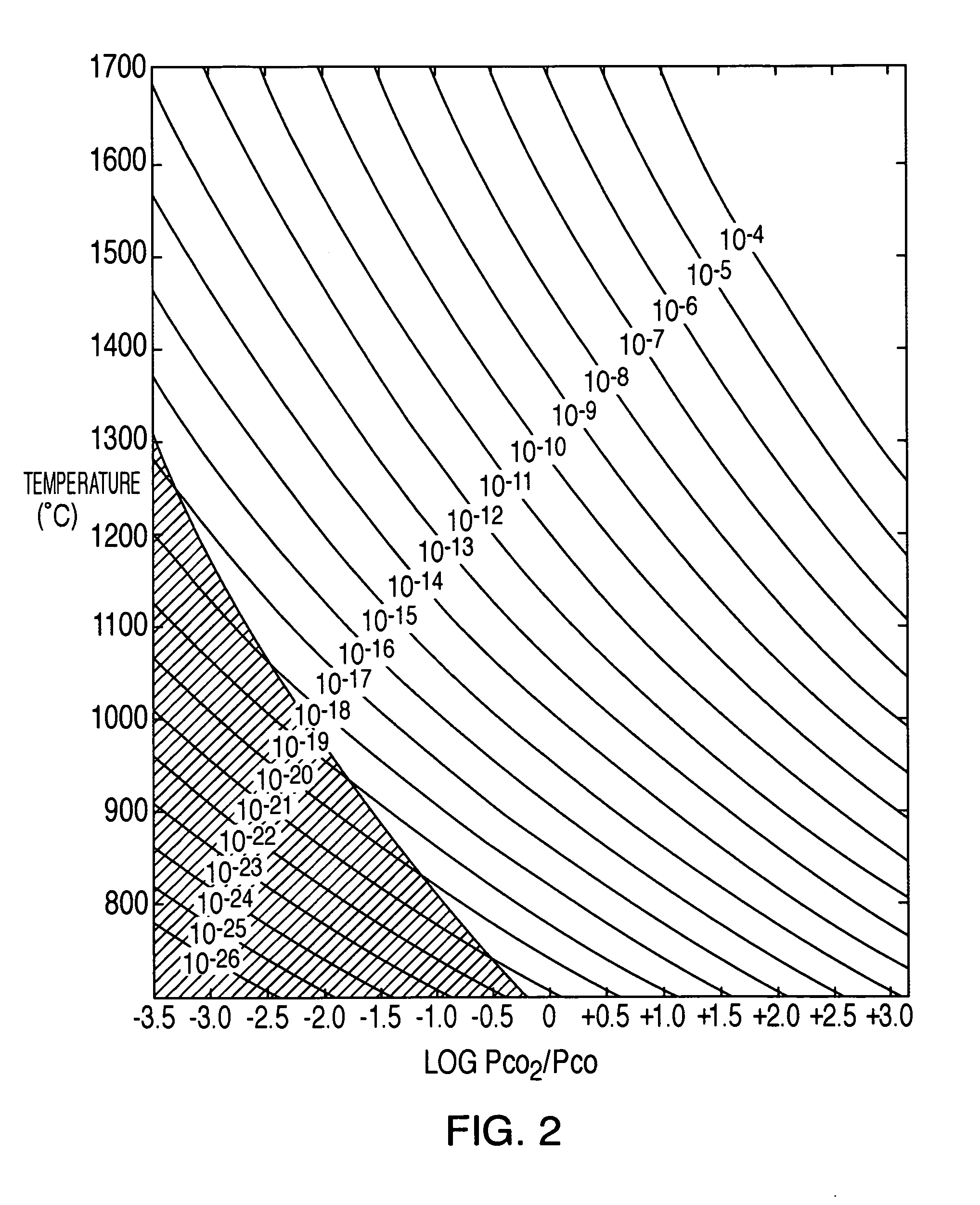
[0021] A 500-gram sample of calcine was charged to a 2 inch (5.1 cm) diameter mini plant fluid bed and heated to 1000° C. The CO2 / CO ratio was controlled at 1000 to affect a pO2 of about 10−8 (See FIG. 2). Fluidizing gas flow rates were 10 L / min N2, 25 L / min CO2 and 0.025 L / min CO. The conditions were maintained for 1 hour and the furnace heater was turned off. The material was allowed to cool rapidly with the gases on. A second reduction test was performed in order to determine the effect of a longer residence time during reduction. The test was conducted under the same conditions but for 2 hours.
[0022] Several bench-scale leaching tests were conducted on the two reduced samples. 25-gram samples of calcine in 250 mL of solution containing 180-g / L of sulfuric acid were utilized. In the first tests, H2O...
example 2
[0031] The effect of iron in a Cu—Ni—Fe system was evaluated. Three samples were tested: 1) Flash Furnace Matte (Cu-23%, Ni-22.6%, Co-0.664%, Fe-25.2%, S-26.7%), 2) Synthetic Matte (Cu-14.9%, Ni-16%, Co-0.435%, Fe-39.2%, S-29.4%), and 3) Bulk Cu—Ni Concentrate (Cu-12.1%, Ni-9.25%, Co-0.278%, Fe-37.5%, S-32.3%) Good separation of copper was achieved in each case. Table 4 summarizes the test results. The following procedure was used with respect to each sample:
[0032] The sample was melted and water granulated. About 2 kg of granulated sample was charged into a refractory pan and heated to ˜900° C. for ˜24 hours in an ambient air atmosphere to provide a roasted material. Fluid bed reduction was effected by first charging 1 kg of the roasted material into a 2 inch (5.1 cm) diameter fluid bed and heating to 1000° C. (external electrical elements) using air for fluidization at ˜45 slpm. The material was allowed to “re-roast” for 10 minutes at 1000° C. and then the air atmosphere was repl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com