Syringe system
a syringe and system technology, applied in the field of syringe systems, can solve the problems of limiting the widespread use of transendomyocardial injection, cell migration and division, and major issue limiting angiogenesis, and achieve the effects of small injection volume, convenient control, and small volum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
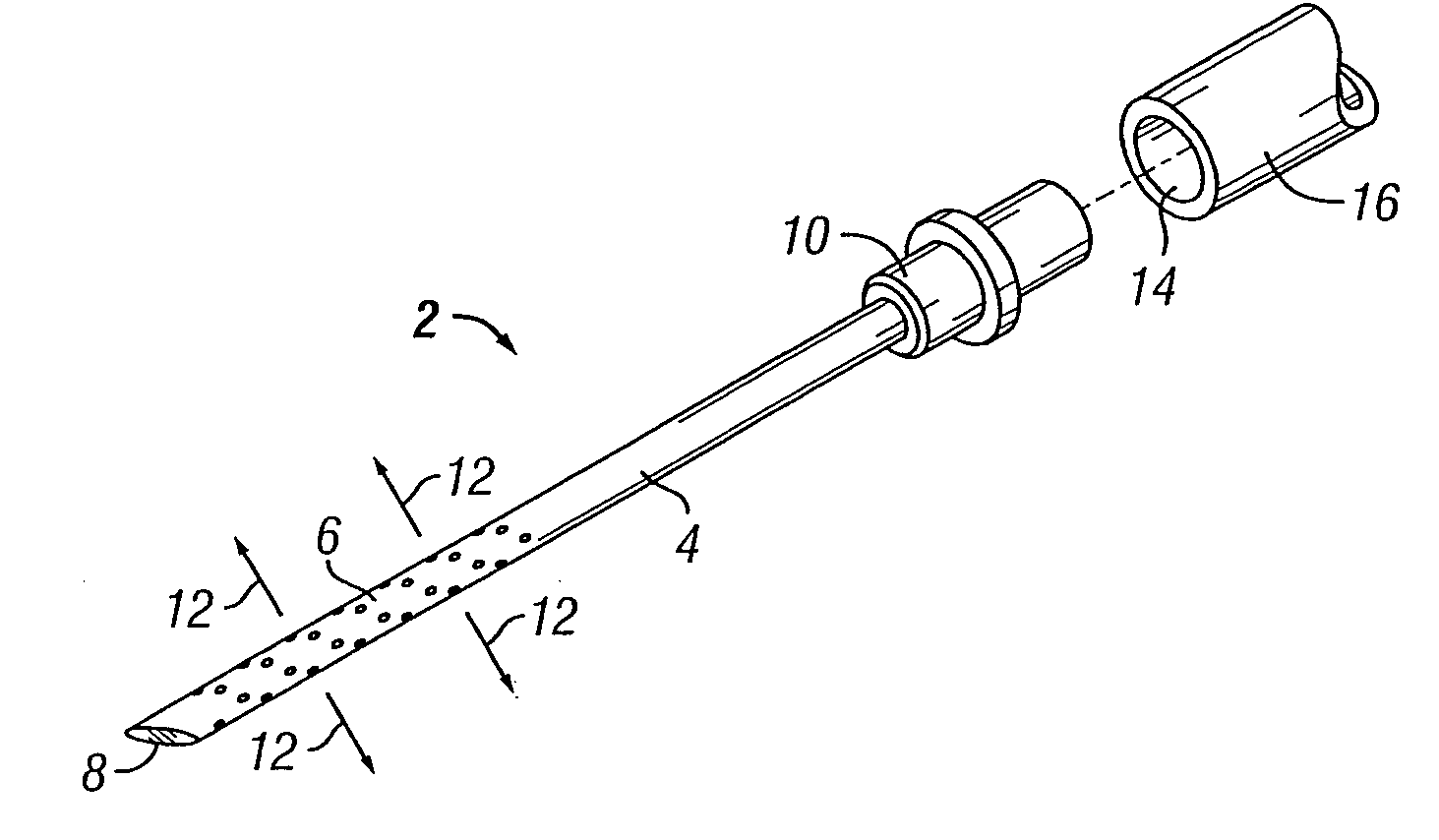
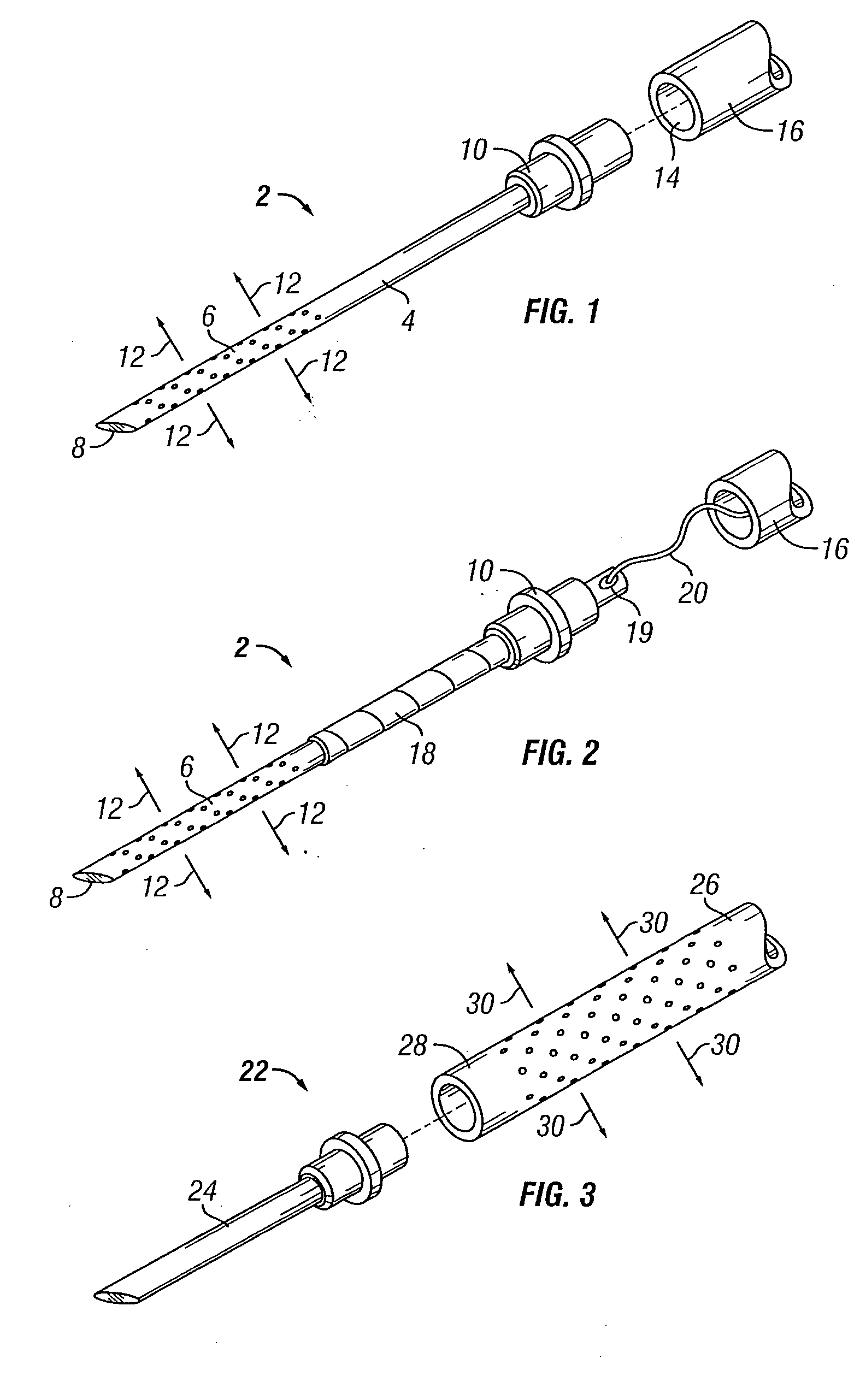
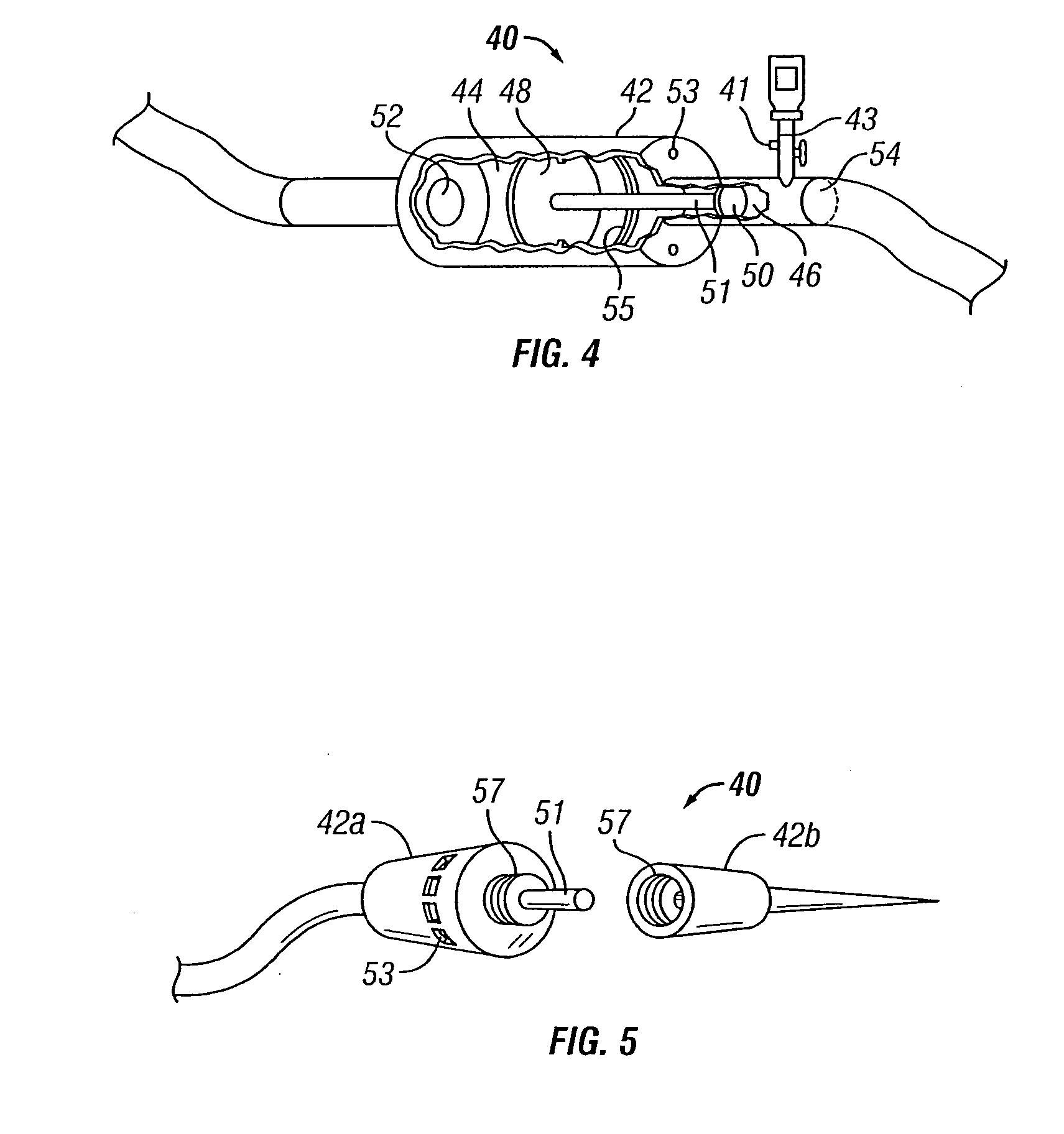
[0068] The present invention overcomes many of the problems in the art by providing a needle and / or a catheter having a plurality of holes formed therethrough for micro or diffused injection of injectates, such as medicaments, living cells, contrast agents, or any liquid, into a body surface. The invention needle comprises a nonporous hollow needle shaft having a proximal end adapted to mate with an injection instrument, a porous or hole-based distal portion in fluid-tight connection to the needle shaft, and a point that is open, closed, or has a solid partial plug. The distal portion of the invention needle is adapted to cause a liquid injectate to weep, ooze, or form any desired 3-dimensional pattern therefrom multidirectionally under injection pressure with the distal portion and point of the needle are inserted into a tissue, chamber, or blood vessel. Typically, the length of the porous distal portion of the needle is determined by its intended use (e.g., whether intended for in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com