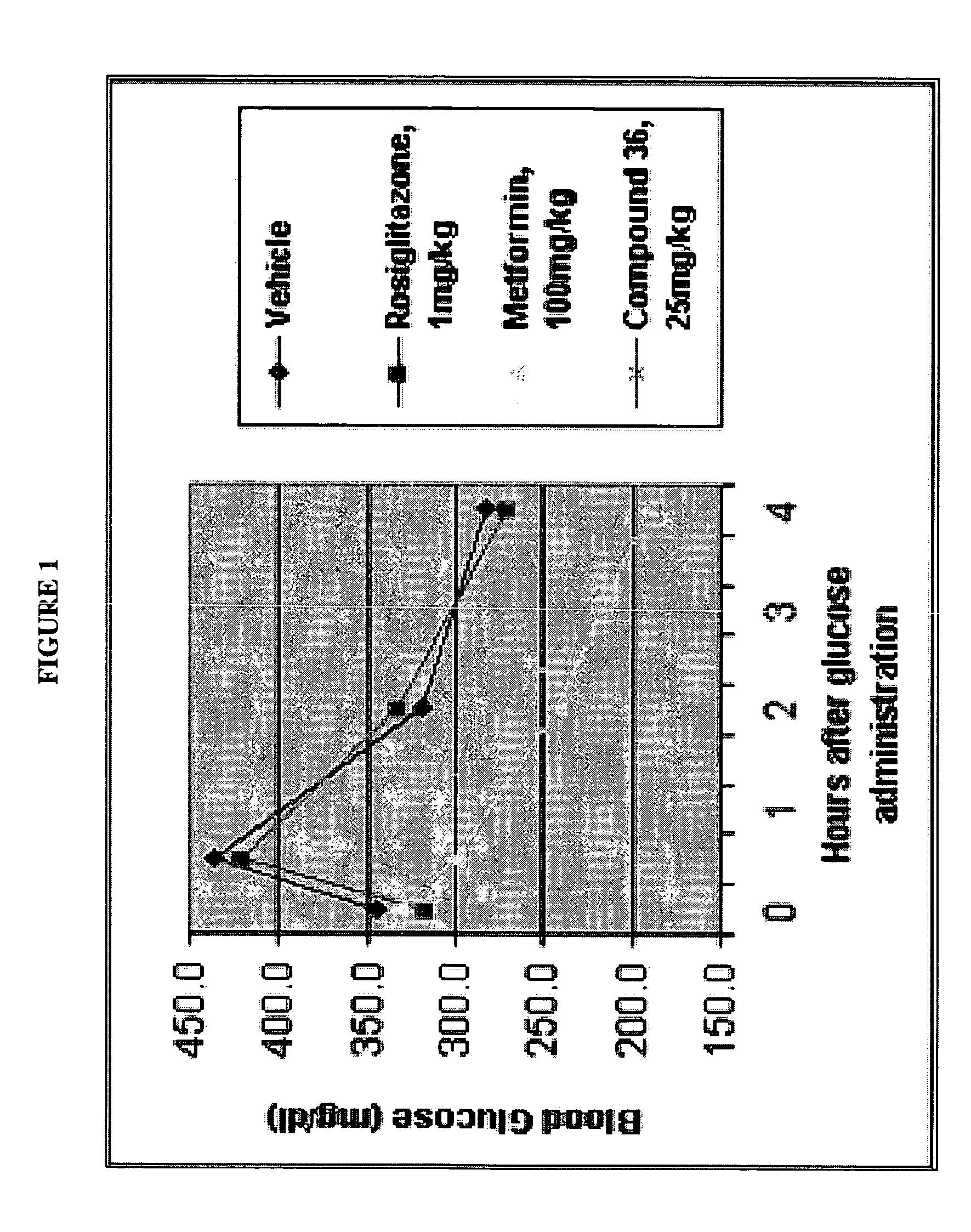
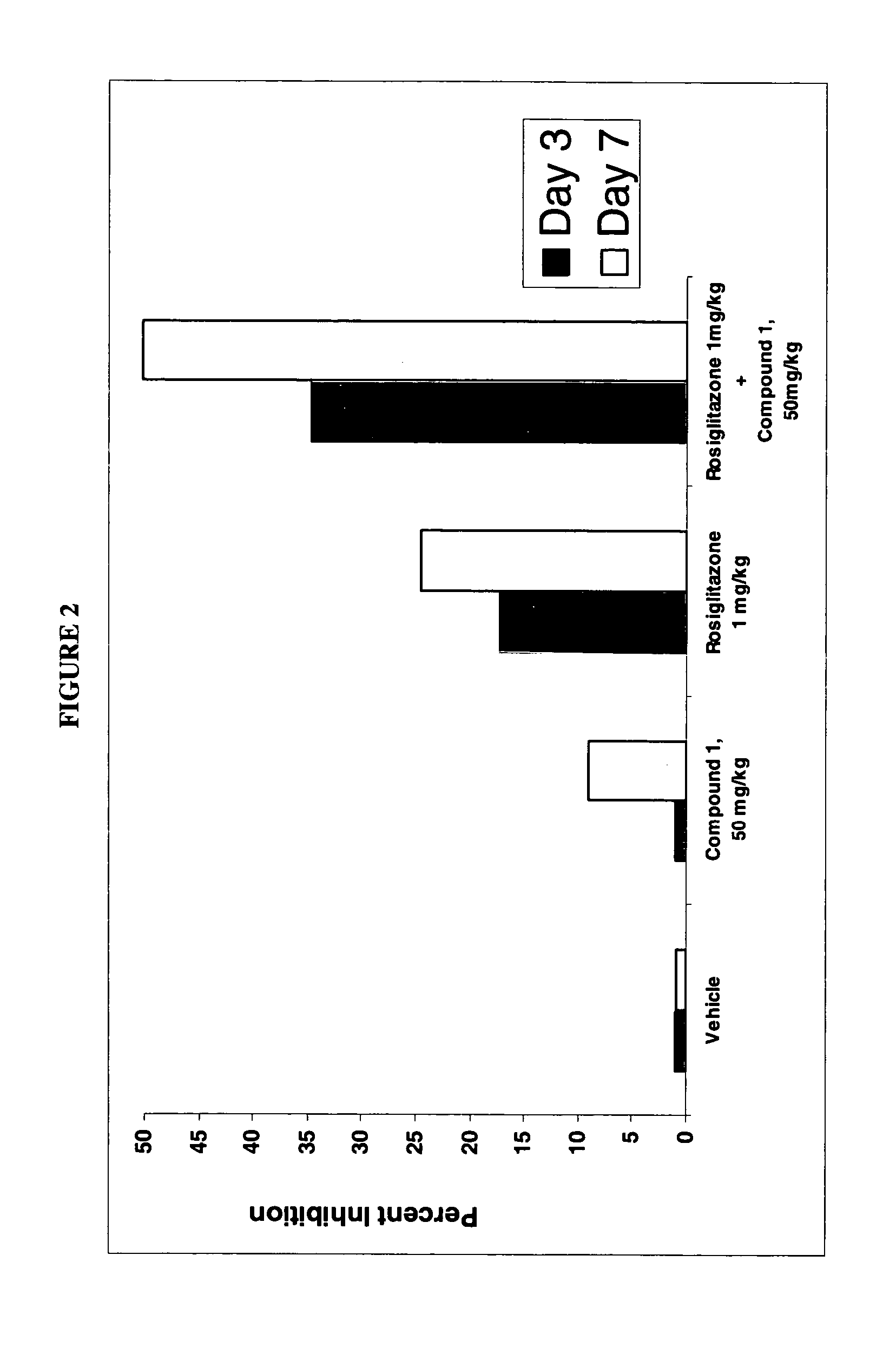
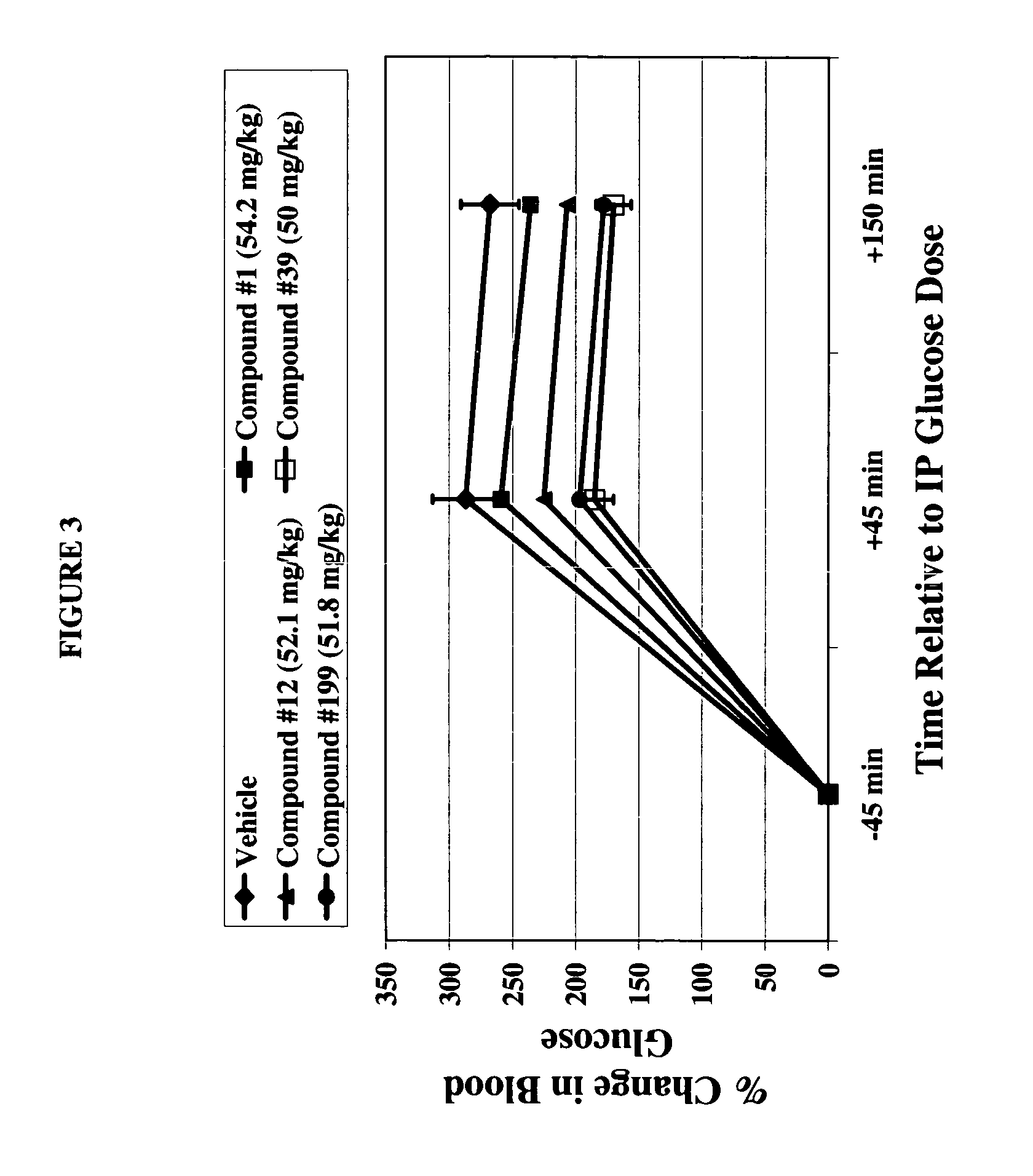
Dihydropyridine compounds for treating or preventing metabolic disorders
a technology of dihydropyridine and metabolic disorders, applied in the field of substituted dihydropyridine compounds, can solve the problems of dangerously high circulating glucose levels, inability to completely produce insulin, and insufficient production of insulin, so as to improve blood glucose levels, improve blood lipid levels, and reduce blood glucose levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 2-(2-azido-ethoxymethyl)-4-(2-chloro-phenyl)-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexa-hydro-quinoline-3-carboxylic acid ethyl ester
[0655] A. 4-(2-azido-ethoxy)-3-oxo-butyric acid ethyl ester
[0656] A solution of 2-azidoethanol (8.7 g, 100 mmol) in THF (20 ml) was added to a suspension of sodium hydride (8.8 g, 220 mmol, 60% dispersion in oil) in THF (150 ml). The mixture was stirred at room temperature for 1 h and then cooled to 0-5° C. Ethyl-4-chloroacetoacetate (16.5 g, 100 mmol) in THF (20 ml) was then added dropwise over a period of 0.5 h. The mixture was stirred at room temperature for additional 16 h and diluted with EtOAc (100 ml) and the pH was adjusted to 6-7 with 2 N HCL. Sufficient water was added to dissolve the solid, and the organic layer was then separated. The aqueous layer was further extracted with EtOAc. The combined organic extracts were washed with brine, dried over MgSO4, filtered and evaporated. The product was purified by column chromatography on si...
example 2
Synthesis of 2-(2-amino-ethoxymethyl)-4-(2-chloro-phenyl)-7,7-dimethyl-5-oxo-1,4,5,6,7,8-Hexahydro-quinoline-3-carboxylic acid ethyl ester
[0659] Compound 1 can be prepared by Route A or Route B, each set forth below.
[0660] A mixture of 2-(2-azido-ethoxymethyl)-4-(2-chloro-phenyl)-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (2.12 g, 4.6 mmol) and tin (II) chloride (2.18 g, 11.5 mmol) in CH2Cl2 / MeOH (2:1, 12 ml) containing 2 drops of water was stirred at room temperature for about 6 h. The reaction mixture was diluted with CH2Cl2 (100 ml) and a saturated solution of NaHCO3 was added to adjust the pH to 9-10, and the layers were then separated. The aqueous layer was further extracted with CH2Cl2. The combined organic extracts were dried over MgSO4, filtered and evaporated. The product was purified by column chromatography on silica gel (CH2Cl2:MeOH, 95:5) to give 2-(2-amino-ethoxymethyl)-(2-chloro-phenyl)-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydro-q...
example 3
Synthesis of 2-(2-amino-ethoxymethyl)-7,7-dimethyl-5-oxo-4-pyridin-2-yl-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester
[0666]
[0667] A solution of 2-azidoethanol (8.7 g, 100 mmol) in THF (20 ml) was added to suspension of sodium hydride (8.8 g, 220 mmol, 60% dispersion in oil) in THF (150 ml). The mixture was stirred at room temperature for about 1 h and then cooled to 0-5° C. Ethyl-4-chloroacetoacetate (16.5 g, 100 mmol) in THF (20 ml) was then added drop wise over a period of about 0.5 h. The mixture was stirred at room temperature for about 16 h and diluted with EtOAc (100 ml) and the pH was adjusted to 6-7 with 2 N HCl. Sufficient water was added to dissolve the solid, and the layers were then separated. The aqueous layer was further extracted with EtOAc. The combined organic extracts were washed with brine, dried over MgSO4, filtered and evaporated. The product was purified by column chromatography on silica gel (Hexane-EtOAc, 95:5) to give 4-(2-azido-ethoxy)-3-ox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar mass | aaaaa | aaaaa |
| Molar mass | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com