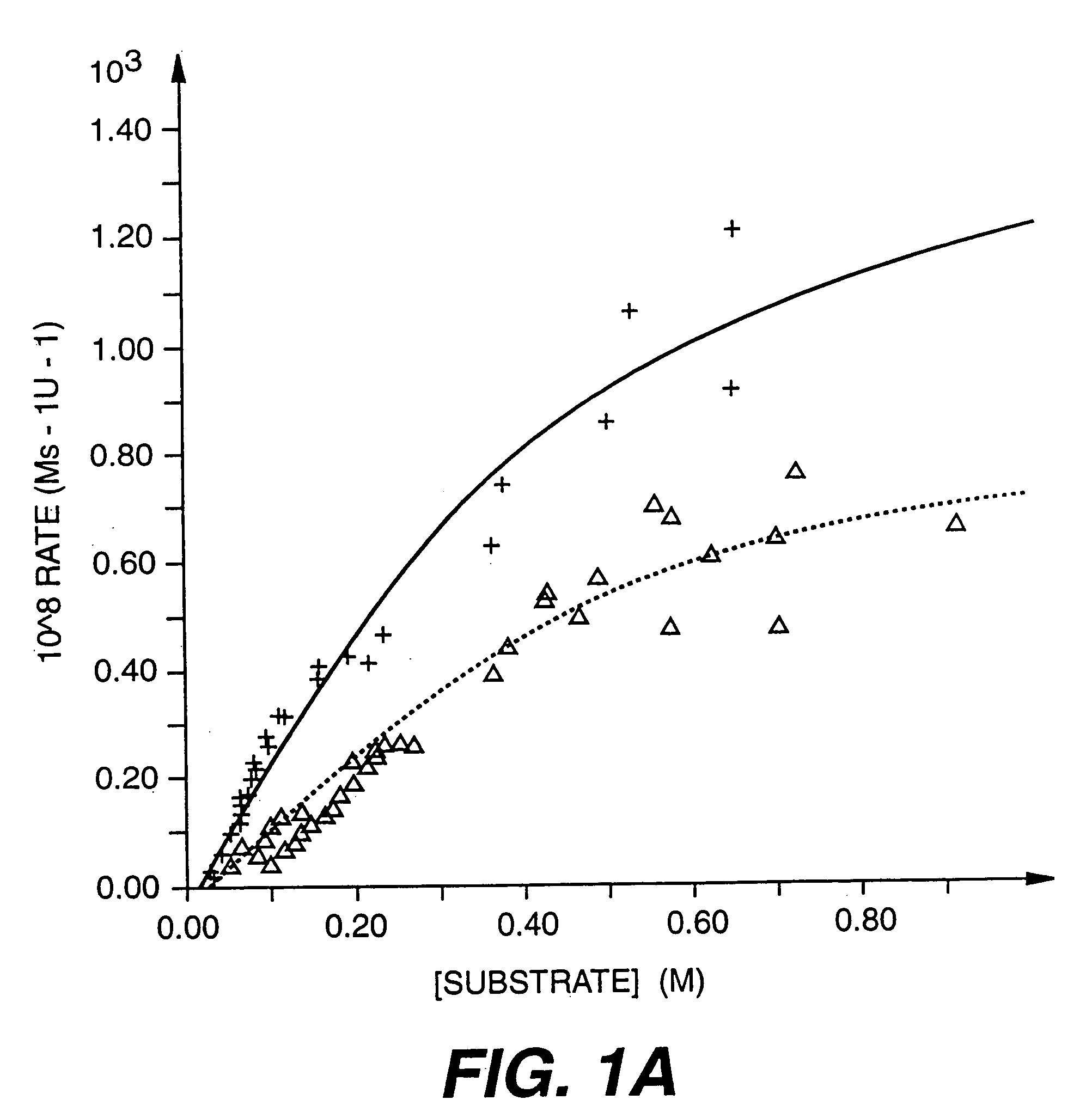
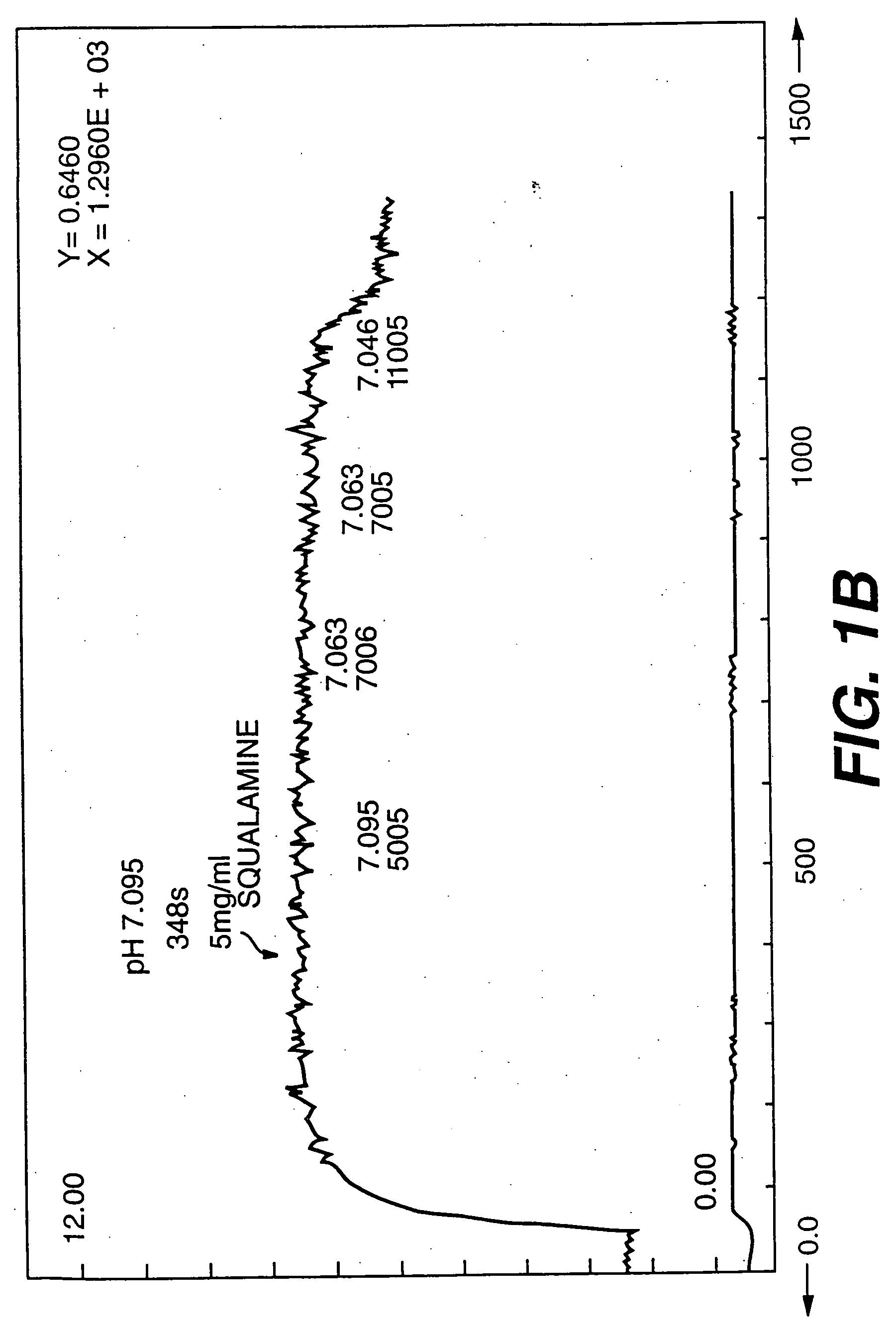
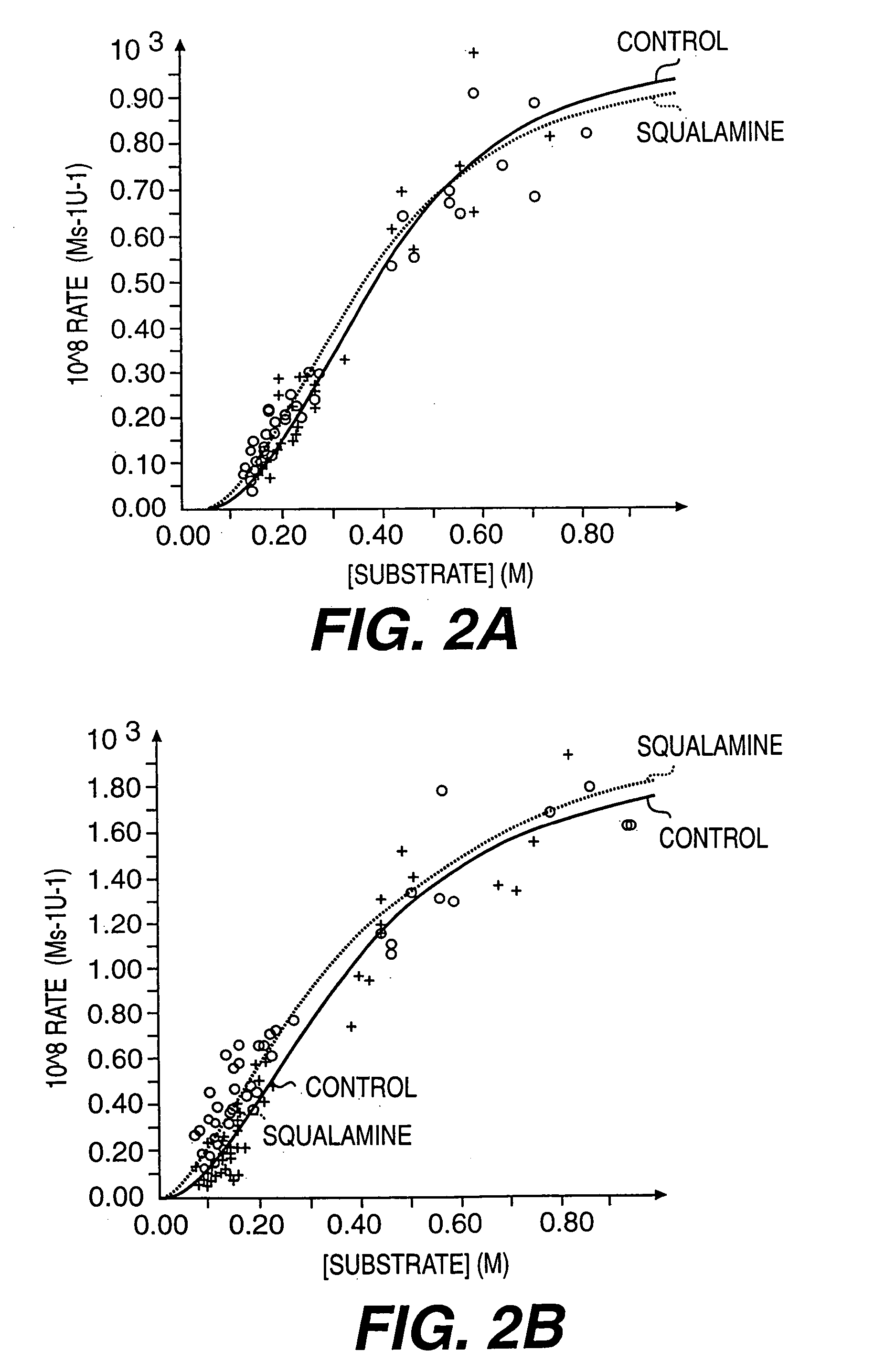
Aminosterol compounds useful as inhibitors of the sodium/proton exchanger (NHE), pharmaceutical methods and compositions employing such inhibitors, and processes for evaluating the NHE-inhibitory efficacy of compounds
a technology of sodium/proton exchanger and aminosterol, which is applied in the direction of instruments, organic chemistry, material analysis, etc., can solve the problems of cell death, decreased contractility, and arrhythmias
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Shark Liver Isolates
[0053] In addition to squalamine, at least ten other distinctly different aminosterols have been recovered from extracts of dogfish shark liver. To prepare the aminosterols, shark liver was extracted in methanol:acetic acid. The aqueous extract was adsorbed to C18 silica and eluted with 70% acetonitrile, and the eluate was adsorbed to SP-sephadex and eluted with 1.5 M NaCl. The eluate was adjusted to 5M NaCl, and the steroids salted out. The precipitate was filtered over Celite and eluted with hot water, followed by methanol. The eluate was reduced in volume and applied to a 1-inch C18 column, and subjected to chromatography utilizing an increasing gradient in acetonitrile. Fractions were collected, concentrated by evaporation, and analyzed separately by thin layer chromatography (TLC).
[0054] The HPLC profile of the aminosterols isolated from 40 kg of shark liver is shown in FIG. 9. Final HPLC purification was performed as described in Moore et a...
example 2
Synthesis of Aminosterols
[0084] In addition to the above compounds, which were isolated from shark liver, synthetic aminosterol compounds have been developed. Various polyaminosterol compounds, including those specified in Examples A-G, are described in U.S. Ser. No. 08 / 416,883, which is the U.S. national phase of International Application No. PCT / US94 / 10265, filed Sep. 13, 1994, the disclosure of which is herein incorporated by reference. Compounds exemplified therein include the following:
[0085] Additional aminosterol compounds have now been developed. Preferred compounds of the invention include those exemplified below.
example h
[0086] Preparation of compound 353 and compound 354:
[0087] The above compounds were prepared by reductive coupling of 5α-cholestan-3-one to spermine (4 equivalents) with sodium cyanoborohydride in a manner analogous to the preparation of compound 303. Purification was achieved on silica gel (gradient elution with 9:3:1 to 3:3:1 chloroform:methanol:isopropylamine). Compound 353 (more polar) and compound 354 (less polar) were converted to their hydrochloride salts in the same manner as for compound 303. α-Amino compound 354: 1H NMR (200 MHz, CD3OD) δ: 3.47 (m, 1H), 3.3-2.9 (m, 12H), 2.3-1.0 (m, 39H), 1.0-0.8 (m, 12H), 0.70 (s, 3H); IR (KBr, cm−1): 3396, 2934, 1594, 1457, 1383; MS(+FAB): 573.6 (M+1); Anal. calcd. for C37H72N4-4HCl—H2O: C=60.31, H=10.67, N=7.60; Found: C=60.01, H=10.83, N=7.67. β-Amino compound 353: 1H NMR (200 MHz, CD3OD) δ: 3.3-3.0 (m, 13H), 2.2-1.0 (m, 39H), 1.0-0.8 (m, 12H), 0.70 (s, 3H); IR (KBr, cm−1): 2945, 1596, 1466, 1383; MS exact mass (+FAB) calcd.: 573.583...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| δ | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com