Multisensor probe for tissue identification
a multi-sensor probe and tissue technology, applied in the field of tissue identification, can solve the problems of high undesirable, invasive, painful and undesirable for patients, and a major handicap for patients in open surgical breast biopsies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
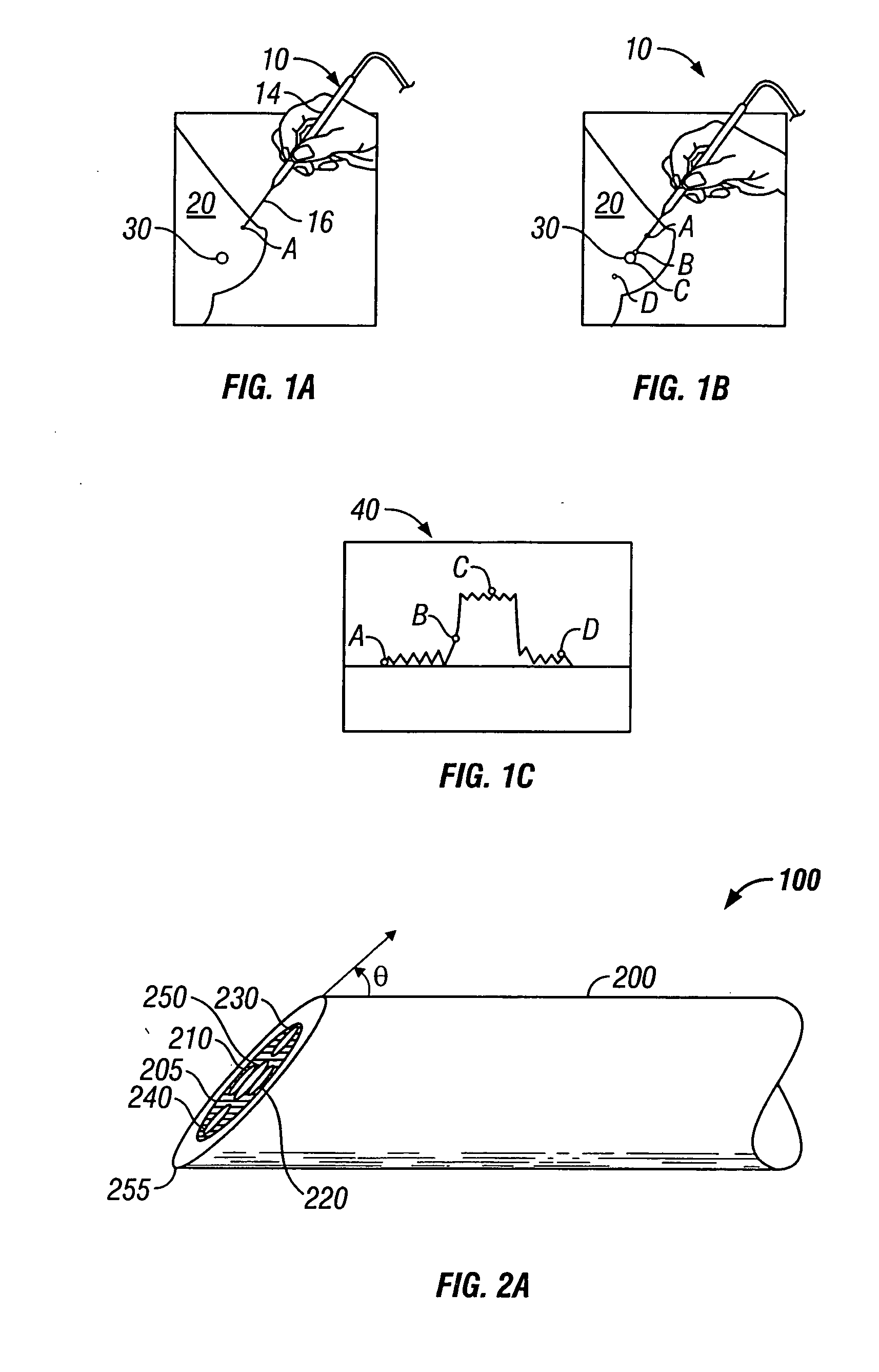
[0040]FIGS. 1A-1C illustrate an embodiment of the present invention in an application. Referring to FIG. 1A, a multisensor probe 10 is shown inserted in breast tissue 20. The multisensor probe 10 includes a handle 14 for manually manipulating the probe and a needle 16 extending from the handle. The distal tip of the needle is shown at location A and is directed towards a suspicious lesion 30. FIG. 1B shows the distal tip of the needle within the suspicious lesion 30 at location C.
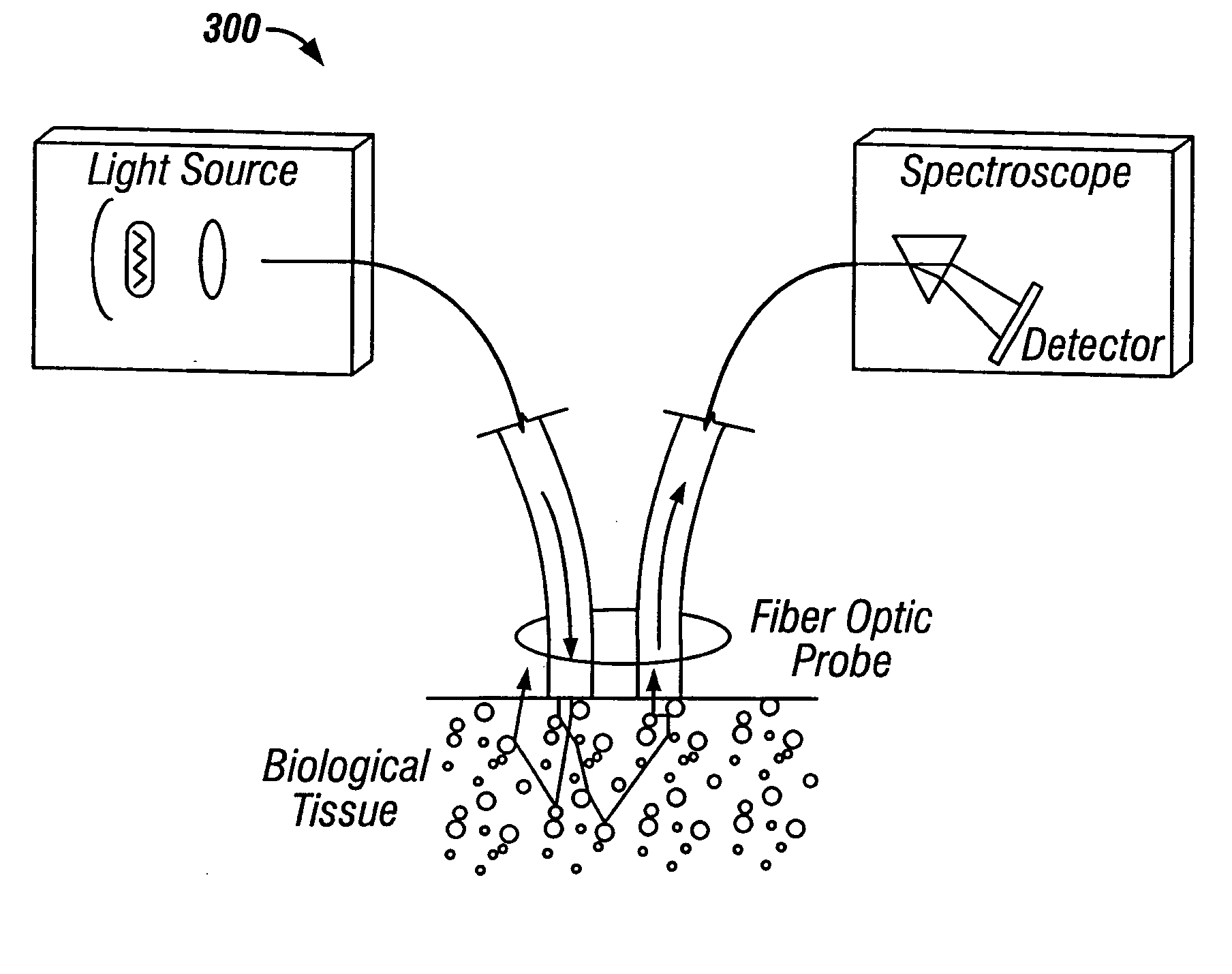
[0041] The probe 10 includes a plurality of sensors to measure tissue properties which are useful in identifying tissue such as cancerous tissue. The sensors may take many forms including, for example, optical fibers for receiving and transmitting light to and from the probe tip. The probe's position or depth is also measured as the probe 10 is inserted into the tissue 20. These measurements are preferably taken and processed continuously and in real time as the probe penetrates the tissue.
[0042]FIG. 1C s...
second embodiment
[0079] Another multisensor probe 600 in accordance with the present invention is shown in FIG. 6. The multisensor probe 600 includes a handle 610 and an elongate body or needle 620 extending from the distal end of the handle. The needle 620 is shown within a slideable sheath 630.
[0080] Sheath 630 is configured such that it retracts into the handle 610 when the needle is inserted into tissue. When not retracted, the slideable sheath 630 covers the needle 620 to protect against accidental needle exposure. The sheath 630 is urged over the needle using a resilient member 660 such as a spring. The spring connects to the sheath and applies a force urging the sheath over the full length of the needle. The force supplied by the resilient member 660, however, is not so great that it inhibits manipulation of the needle into the tissue. The resilient member is thus selected or adjusted to allow the sheath to easily retract as the needle is inserted into tissue. Suitable materials for the shea...
examples
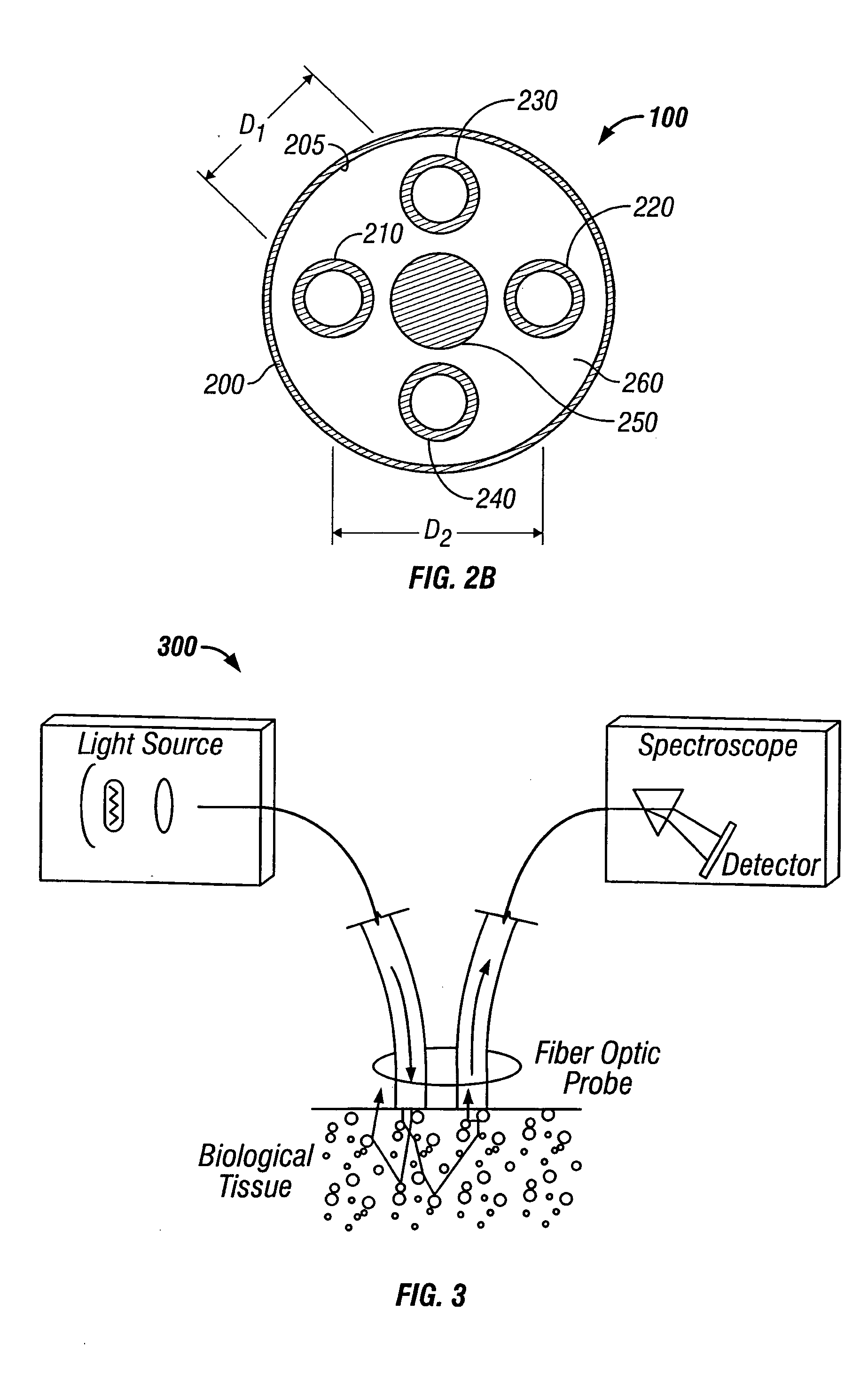
[0120] A multisensor probe in accordance with the present invention was built and tested. The probe featured a needle, a handle for manipulating the handle, an OSAS sensor, and OCDR sensor, and an impedance sensor. The OSAS sensor included a source fiber and two collection fibers. The OCDR sensor included a single mode fiber. The electrical impedance sensor included a central conductor as one electrode and the outer needle wall as the second electrode.
[0121] A xenon flash lamp was used as a light source for the test probe. FIGS. 11A and 11B show the spectrum of light collected by two OSAS fibers during in-vitro testing for normal and malignant tissue respectively. The line or “signature” represented by “channel 2” represents light collected from one optical fiber and the line represented by “channel 3” represents light collected from another optical fiber. The “channel 2” optical fiber was closer (center to center) to the light emitting fiber than the “channel 3” fiber.
[0122] Ampl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com