Method of determining analyte level using subcutaneous electrode
a technology of subcutaneous electrodes and analyte levels, which is applied in the field of in vivo enzyme biosensors, can solve problems such as loss of sensitivity, and achieve the effect of accurate measuremen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
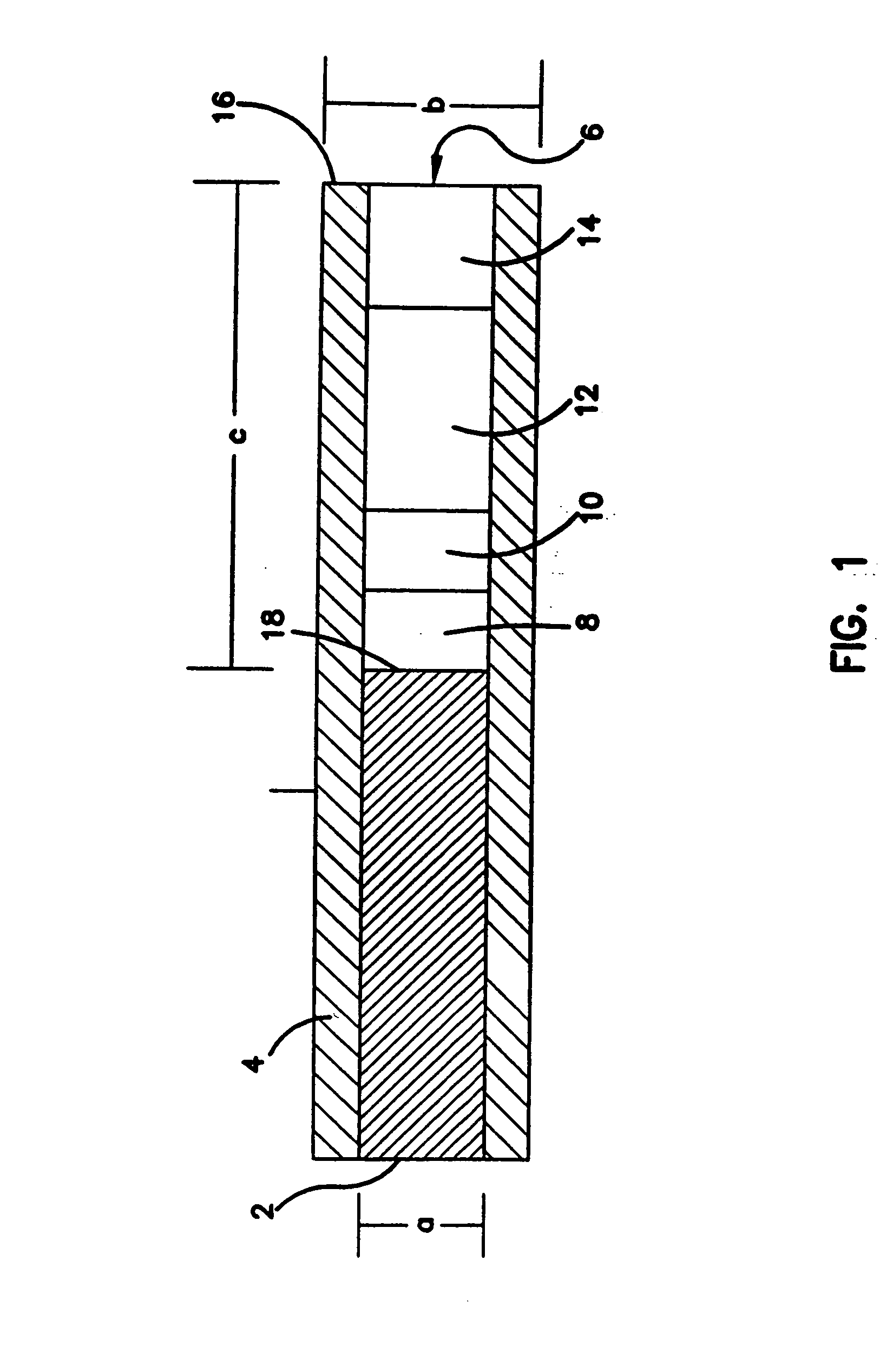
[0055] Electrode Preparation
[0056] Electrodes were made of a polyamide-insulated 250 μm diameter gold wire, having an outer diameter (O.D.) of 290 μm (California Fine Wire Co., Grover City, Calif.). Heat shrinkable tubing (RNF 100 3 / 64″ BK and 1 / 16″ BK, Thermofit.RTM., Raychem, Menlo Park, Calif.) and a two component silver epoxy (Epo-tek H2OE; Epoxy Tech, Inc., Billerica, Mass.) were used for electrode preparation.
[0057] The glucose sensing layer was made by crosslinking a genetically engineered glucose oxidase (rGOX) (35% purity, Chiron Corp., Emeryville, Calif.) with a polymer derived of poly(vinylimidazole) (PVI), made by complexing part of the imidazoles to [Os(bpy)2C]+ / 2+. The resulting redox polymer, termed PVI-Os, was synthesized -according to a previously published protocol. (Ohara et al., 1993, Anal. Chem., 65:24). Poly(ethylene glycol) diglycidyl ether 400 (PEDGE; Polysciences, Warrington, Pa.) was used as the crosslinker.
[0058] The barrier layer between the sensing an...
example 2
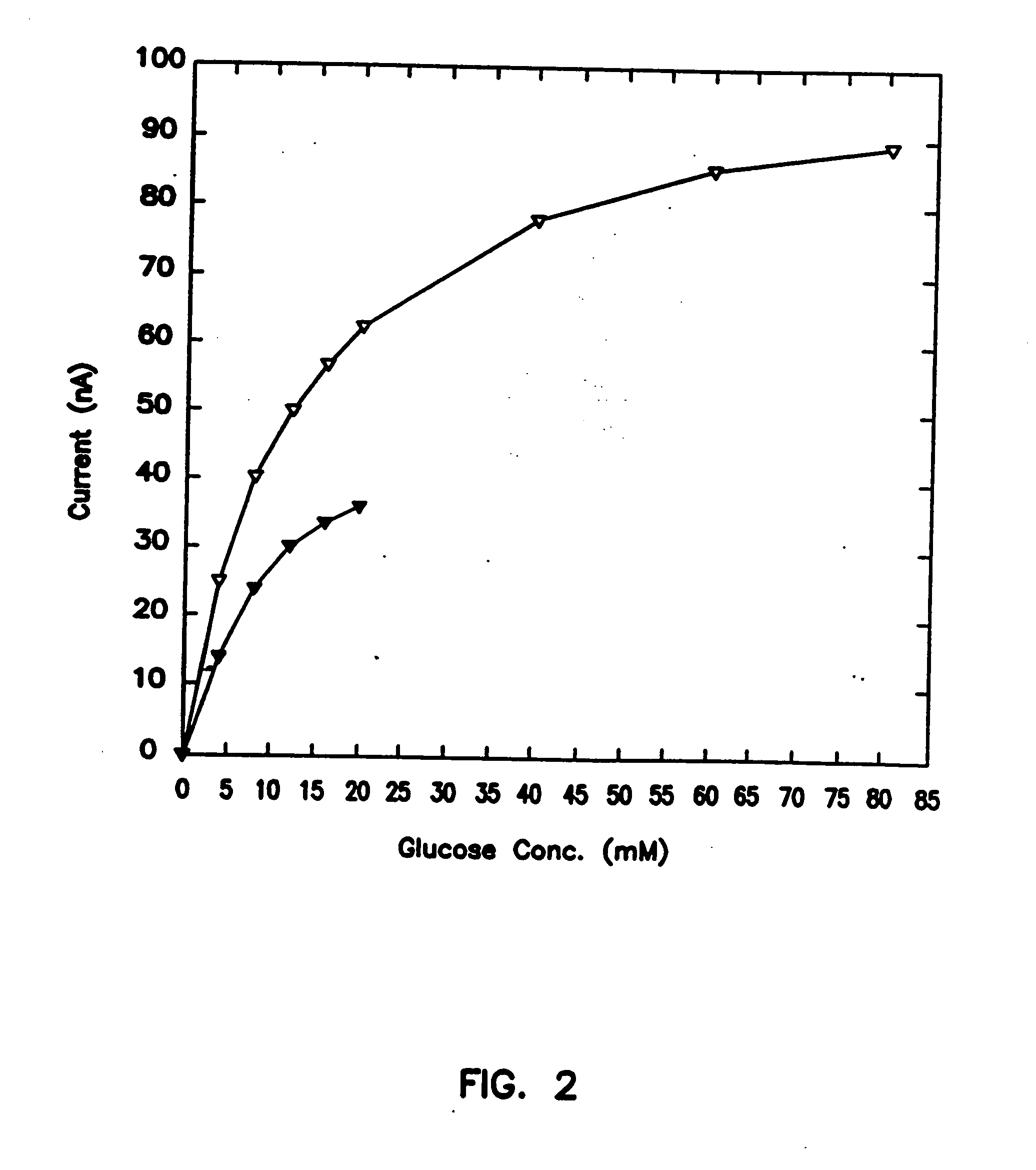
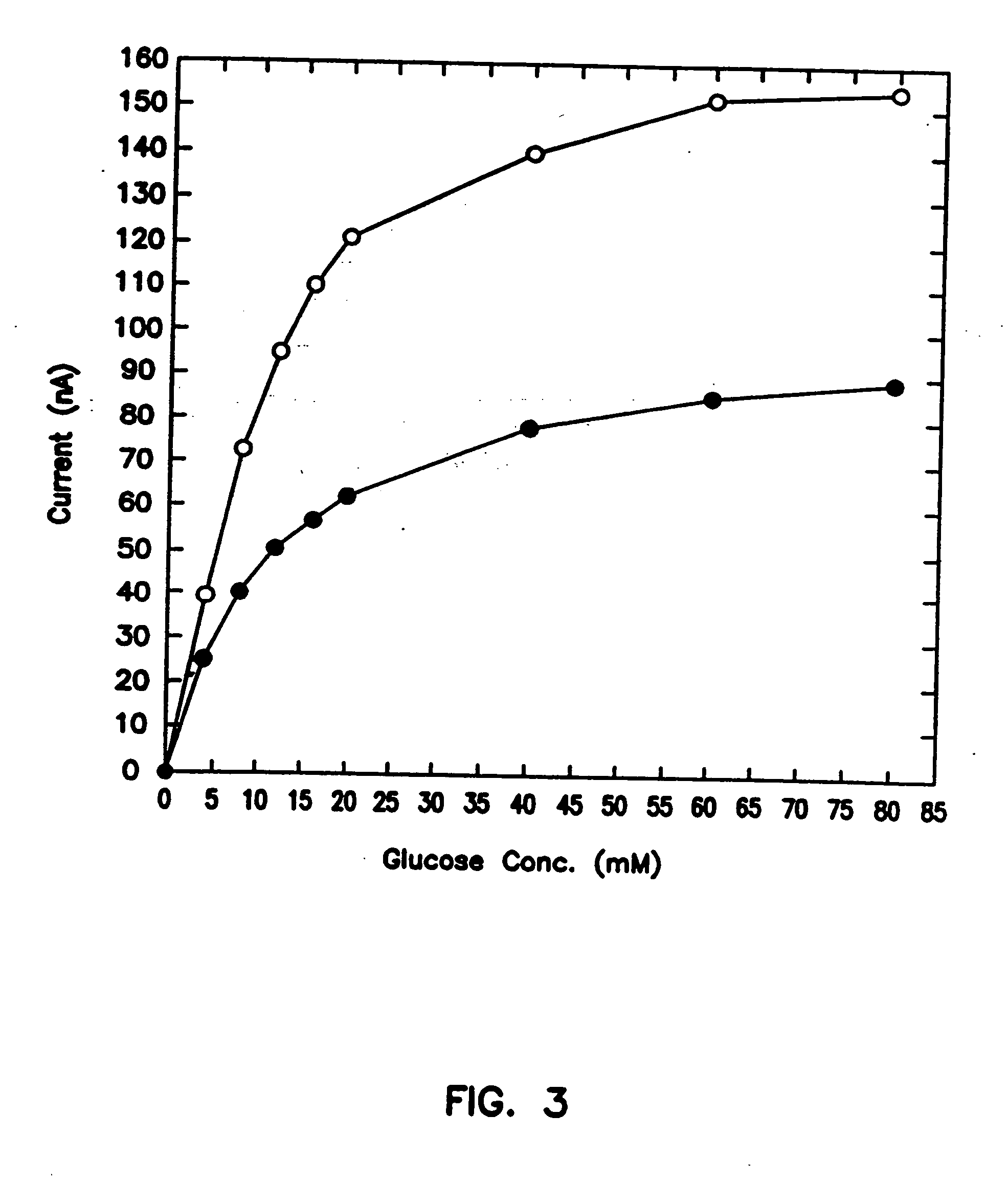
[0088] In Vivo Use of Sensor
[0089] The objective of this experiment was to establish the validity of a one-point in vivo calibration. Two sensors were simultaneously implanted subcutaneously in a rat, one on the thorax, the second between the scapulae. To make the difference between the blood sampled and the subcutaneous fluid proved with the sensors as extreme as possible, i.e., to probe whether the one-point calibration holds even if the organs sampled are different and the sampling sites are remote, blood was withdrawn from the tail vein. Blood glucose levels were periodically measured in withdrawn samples, while the absolute uncorrected sensor current output was continuously monitored.
[0090] In vivo experiments (6-10 hours) were carried out in 300 g male Sprague-Dawley rats. The rats were fasted overnight and prior to the experiment were anaesthetized with an intraperitoneal (i.p.) injection of sodium pentobarbital (65 mg / kg rat wt). An i.p. injection of atropine sulfate (166 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com