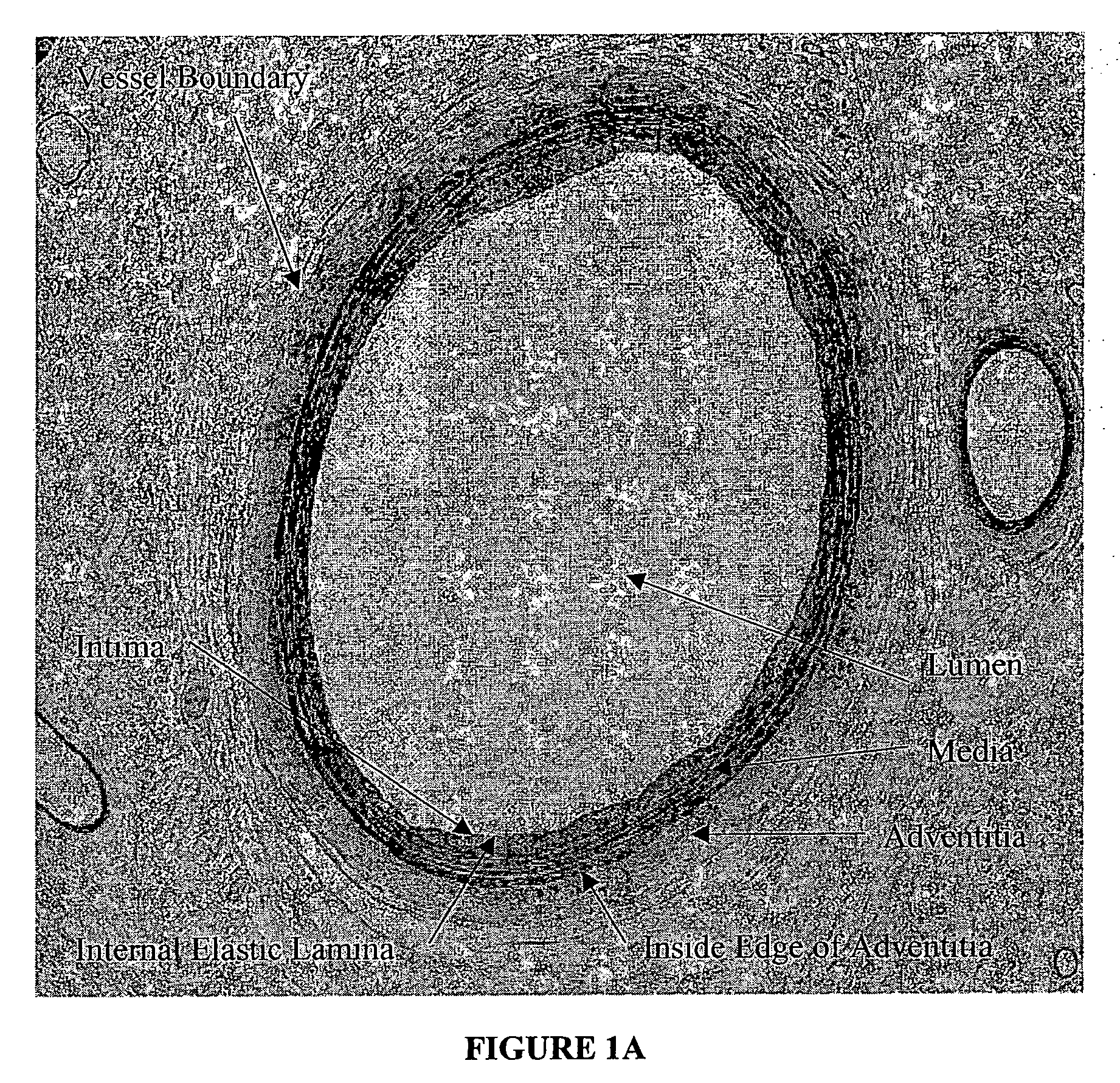
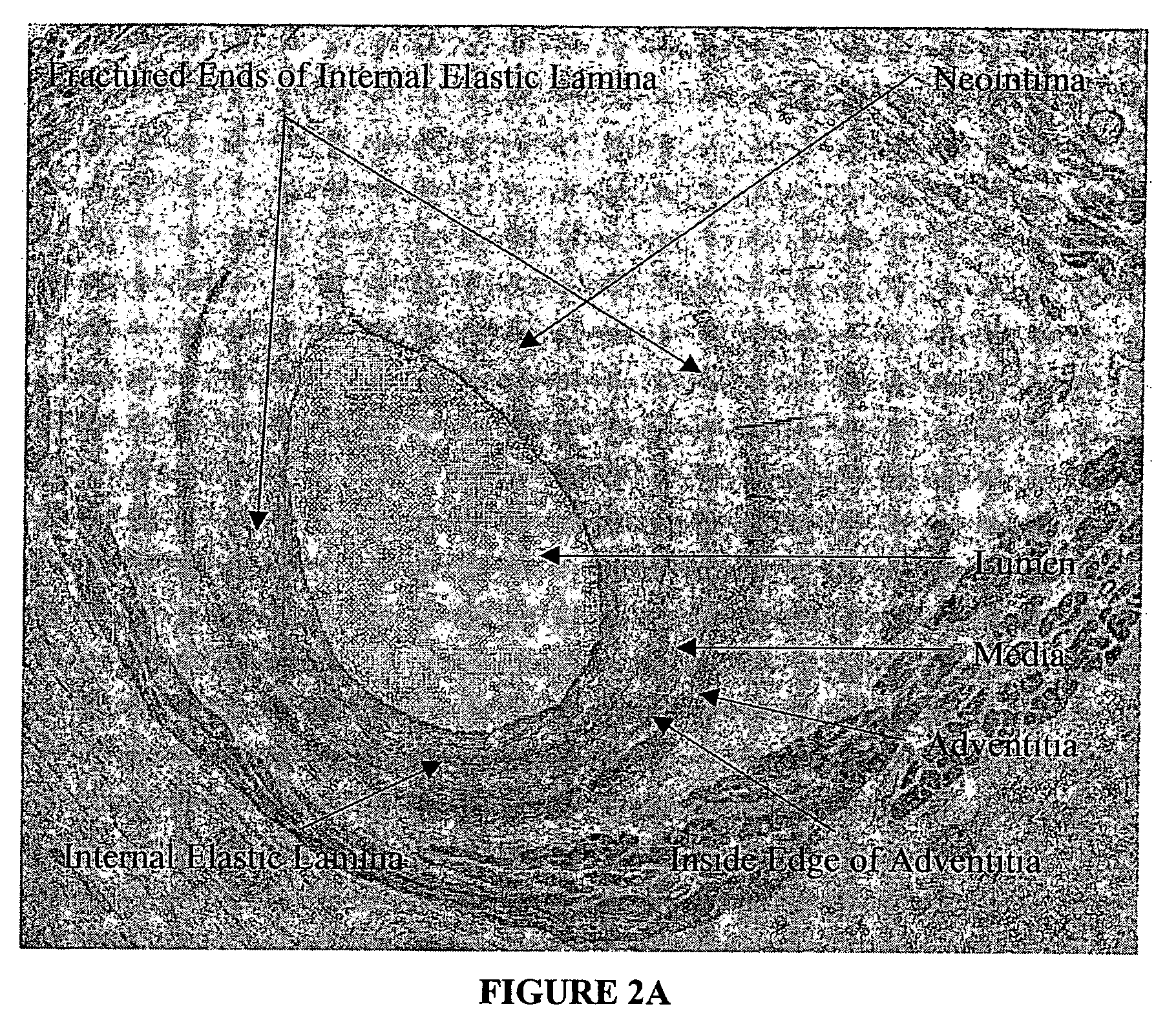
Method for quantitative analysis of blood vessel structure
a blood vessel and quantitative analysis technology, applied in the field of tissue characterization, can solve the problems of inability to accurately determine whether there is any detectable difference, manual process cannot be replicated exactly from one time to the next, and the contours of feature boundaries of dozens of vessel specimens a week are tedious and laborious, so as to reduce the amount of tedium involved, accurate and repeatable analysis of the shape of components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] In the Applicant's co-pending U.S. patent applications Ser. Nos 09 / 338,904, No. 09 / 338,909 and No. 09 / 338,908, novel databases having structural, cell function and / or mechanical indices that correspond to statistically significant representations of tissue characteristics are disclosed
[0027] The present invention relates to a novel approach to an automated measurement and analysis system to quantitatively evaluate tissue structural indices and shape of tissue specimens (for example, cross sections of tissues or organs). The tissue specimens that can be analyzed by the present invention include liver, kidney, bile duct, gastrointestinal tract, lymphatic vessel, bronchia and blood vessels. The section (e.g., cross section) of a given tissue specimen does not necessarily exclude the presence of tissue that is naturally present surrounding the given tissue specimen under study. For example, blood vessels are present in muscle tissue. A cross section of a given blood vessel from ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com