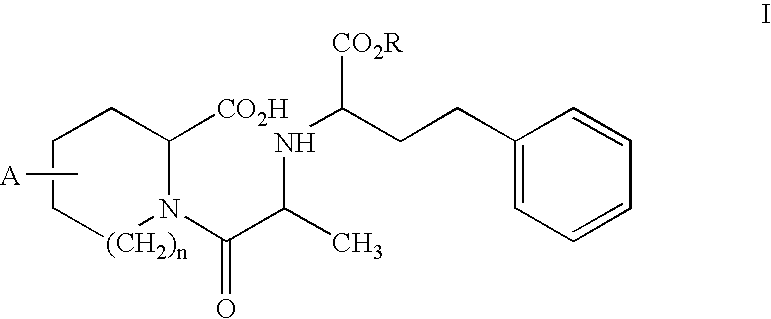
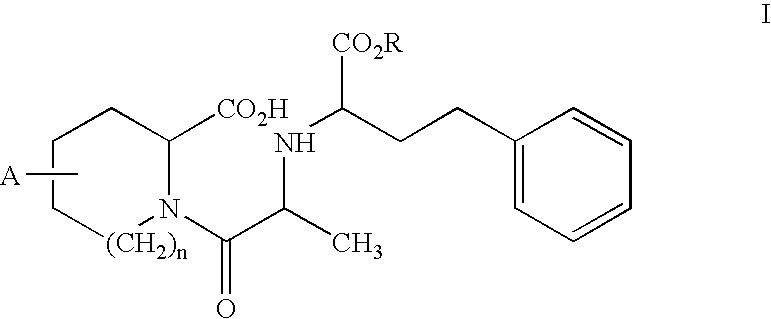
Stabilization of quinapril using magnesium oxide
a technology of magnesium oxide and quinapril, which is applied in the field of stabilization of quinapril with magnesium oxide, can solve the problems of magnesium carbonate hydroxide being white, difficult to form tablets, and difficult to meet the requirements of wet granulation process labor and cost, so as to minimize the cyclization degradation of ace inhibitors, improve the formulation of ace inhibitors, and minimize the hydrolysis of ace inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0075] The following materials were processed by wet granulation method for the manufacture of 20-mg tablets.
Quinapril Hydrochloride21.7 mgMagnesium Oxide21.7 mgLactose254.3 mg Gelatin 6.4 mgPolyplasdone12.8 mgMagnesium Stearate 3.2 mg
example 2
[0076] The following materials were processed by wet granulation for the manufacture of 5-mg tablets without the addition of a stabilizer.
Quinapril Hydrochloride5.425 gLactose Anhydrous119.575 g Microcrystalline Cellulose14.775 g Disodium EDTA0.225 gSterotex HM1.500 gSyloid 244 Silica Gel3.000 gStearic Acid4.500 gAscorbic Acid USP1.000 gWater, Purified USP2.250 g
example 3
[0077] The following materials were processed by wet granulation for the manufacture of 20-mg tablets without the addition of a stabilizer of the present invention.
Quinapril Hydrochloride21.7 mgMagnesium Carbonate Hydroxide125.0 mg Lactose33.3 mgGelatin10.0 mgPolyplasdone 8.0 mgMagnesium Stearate 2.0 mg
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com