Tissue augmentation material and method
a tissue and augmentation technology, applied in the field of biocompatible compositions for soft tissue augmentation, can solve the problems of unsatisfactory voice quality, respiratory obstruction from over-injected teflon®, etc., and achieve the effect of improving the delivery of augmentation materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Gel
[0100] A mixture of 15% glycerin, 85% water, (based on the combined weight of the water and glycerin) and 3.25% NaCMC (again based on the total of the liquid components) is prepared in the following manner:
[0101] 9.303 g of glycerin and 2.016 g of NaCMC are combined in a vessel. The mixture is then slowly added to 52.718 g of agitating water in a container large enough for batch size and allowed to mix, utilizing an electric mixer, for 30 minutes at a medium speed. The gel is allowed to set for a minimum of four hours.
example 2
Preparation of the Augmentation Composition
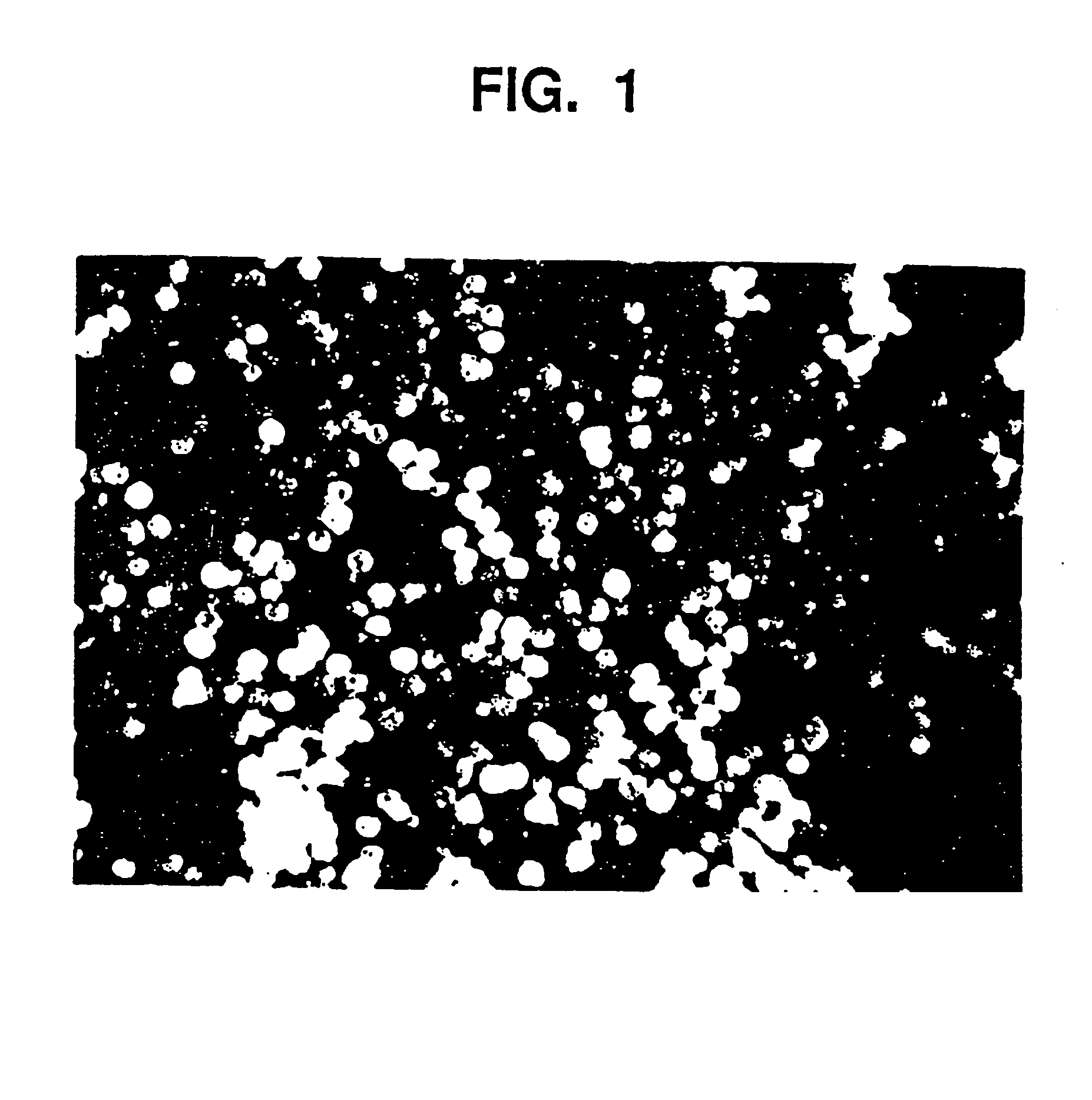
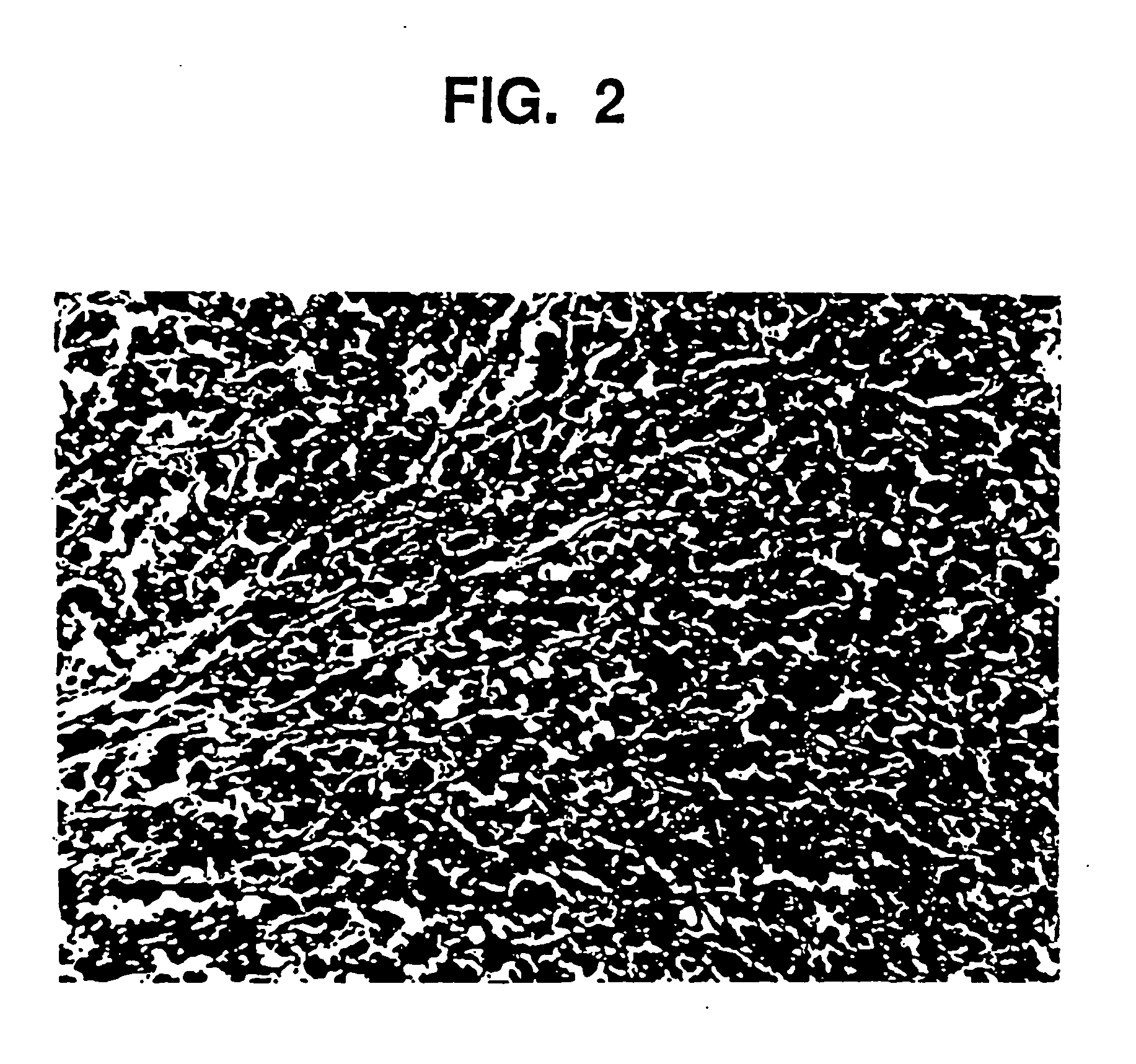
[0102] Aqueous glycerin / NaCMC gel (44.04 g, prepared in Example 1) are placed in a mixing container large enough for batch size. Smooth, rounded substantially spherical CaHA particles (55.99 g) having a uniform particle size of 75 to 125 microns are thoroughly blended, utilizing an electric mixer, for five minutes at a low speed until all the particles are homogeneously distributed in a uniform suspension in the gel. The blended material is packaged in 3 cc polysulfone cartridges and sterilized in an autoclave for 60 minutes at 121° C.
example 3
Properties of Augmentation Composition
[0103] The gel as prepared in Example 1, and the augmentation medium as prepared in Example 2 are examined by means of a parallel plate rheometer (Häake RS100). Testing includes the measurement of Theological properties as a function of applied stress (stress ramp), deformation at constant stress followed by recovery at 0 stress (creep / recovery), and the measurement of the complex modulus using an oscillating stress within the viscoelastic limit of the composition (frequency sweep). Outcomes demonstrate that the behavior of the gel and augmentation composition, both before and after sterilization, is the same. For example, this is demonstrated in FIG. 3, which shows the viscosity of the gel and augmentation material before and after sterilization as a function of applied stress from 10 to 1000 Pascals. The shape of the curves are similar and demonstrate the shear thinning characteristic of this material. Other measured values are given in the f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle sizes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com