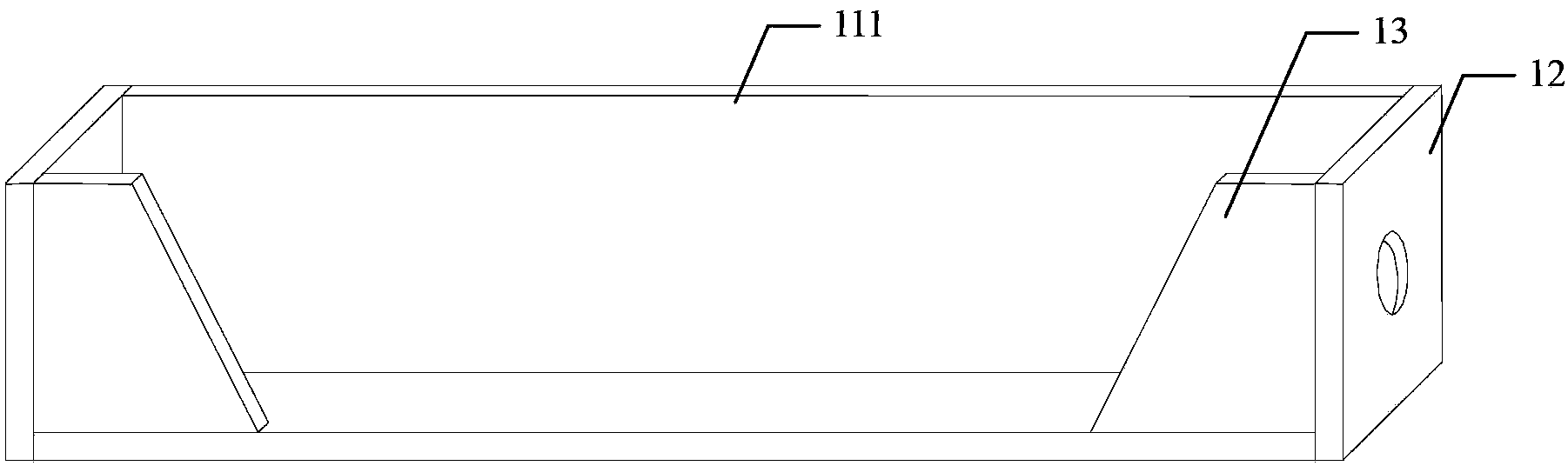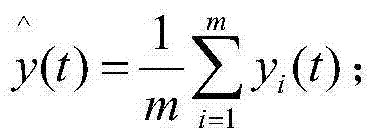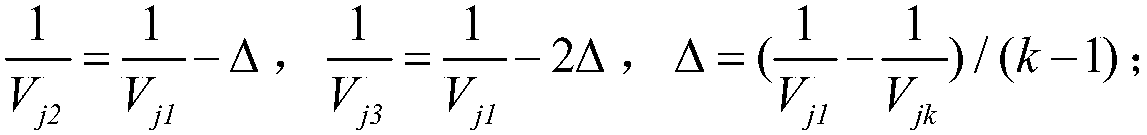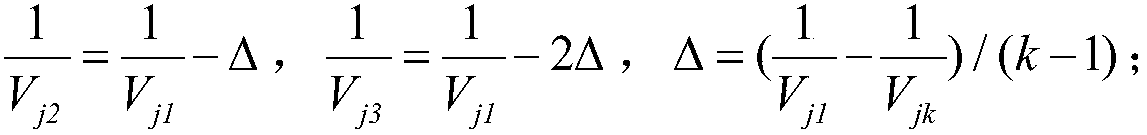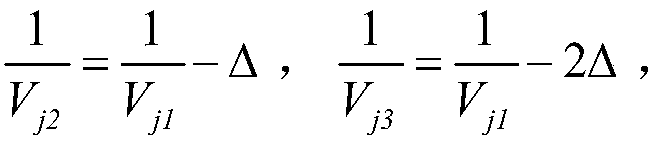Patents
Literature
136 results about "Constant stress" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Constant stress can affect your sleep patterns and make you irritable and fatigued, unable to concentrate and highly reactive. You may become unable to relax and operate in a state of anxiety. Depression is a common reaction to chronic stress.
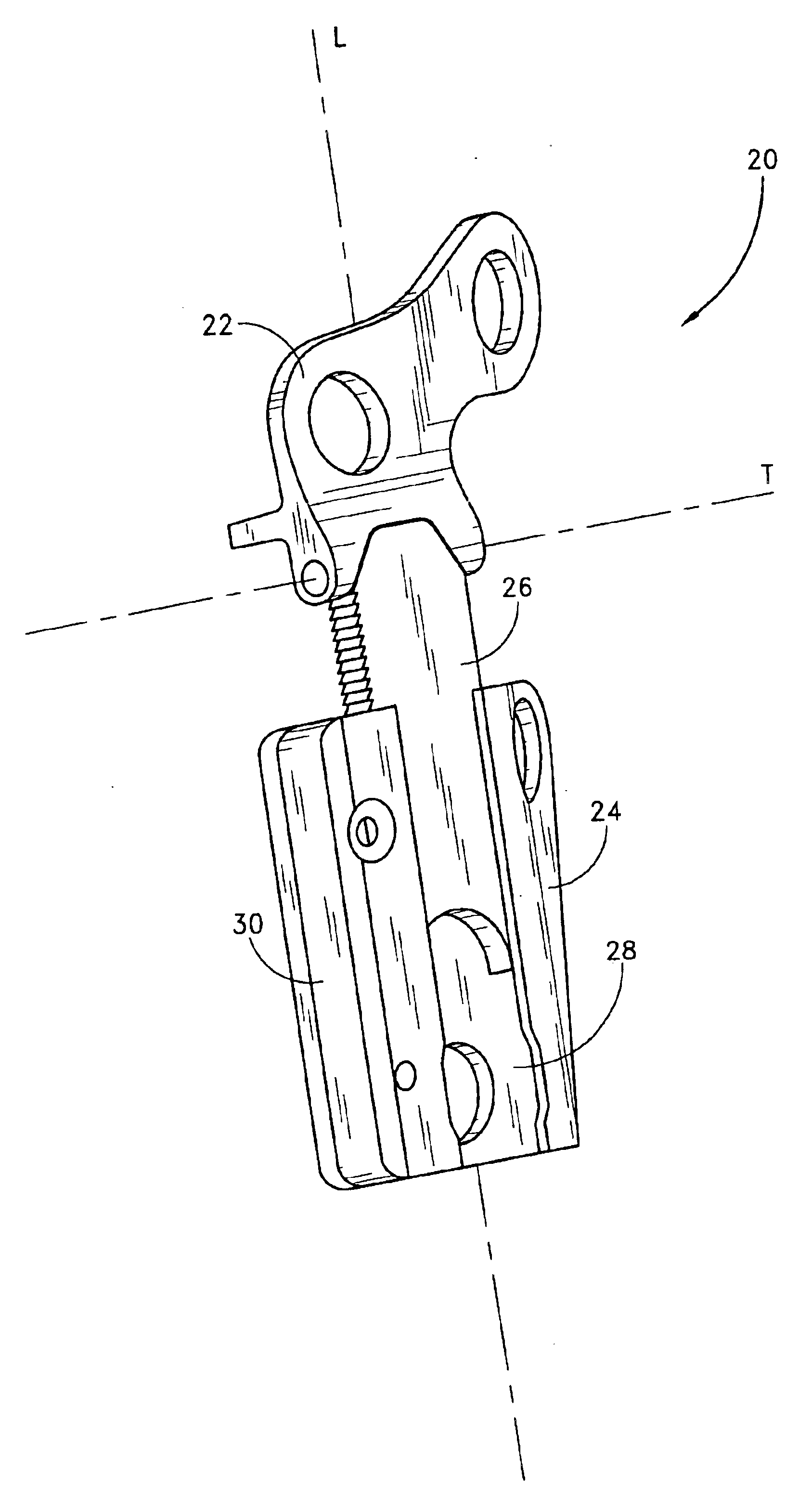
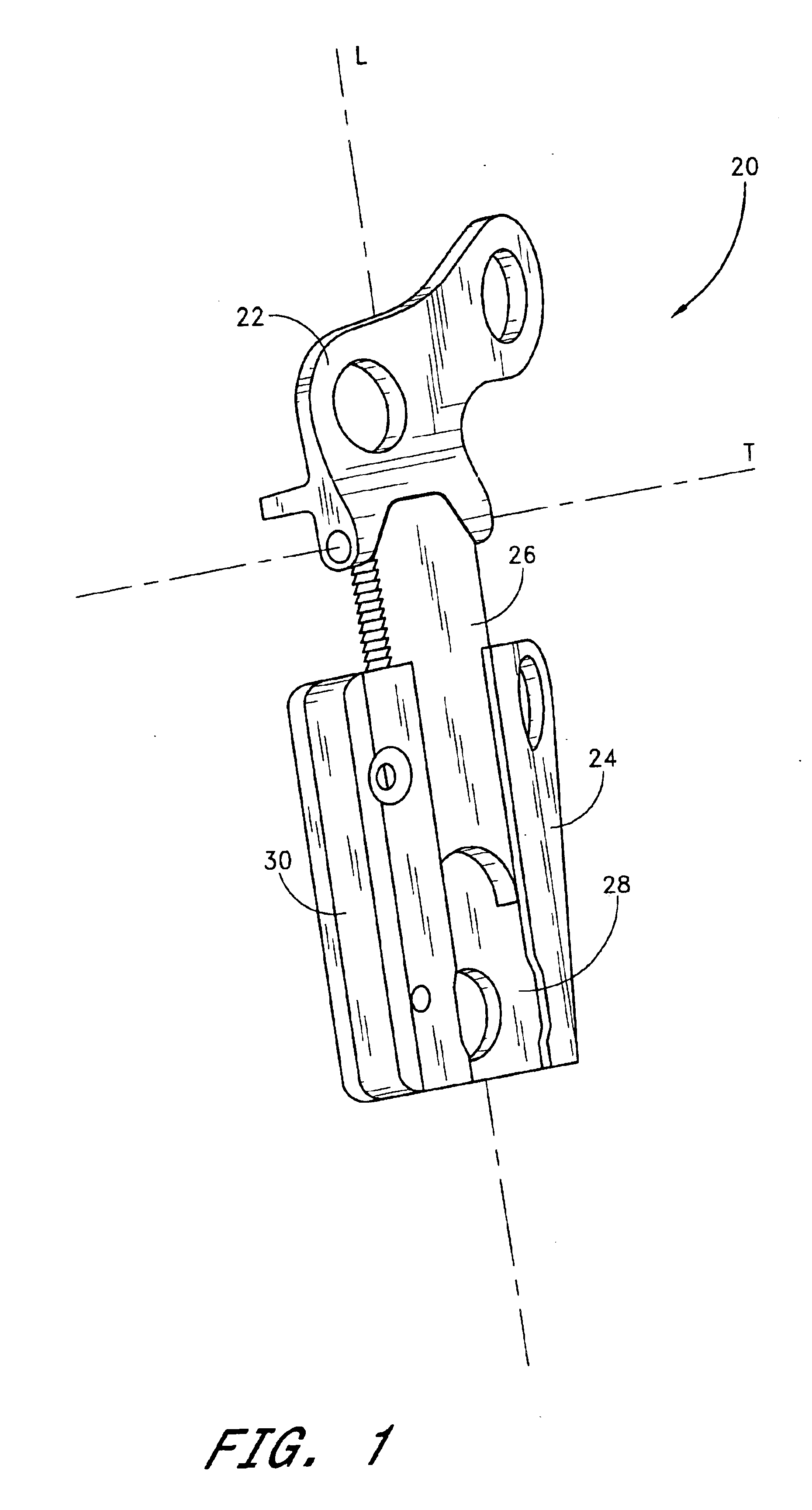
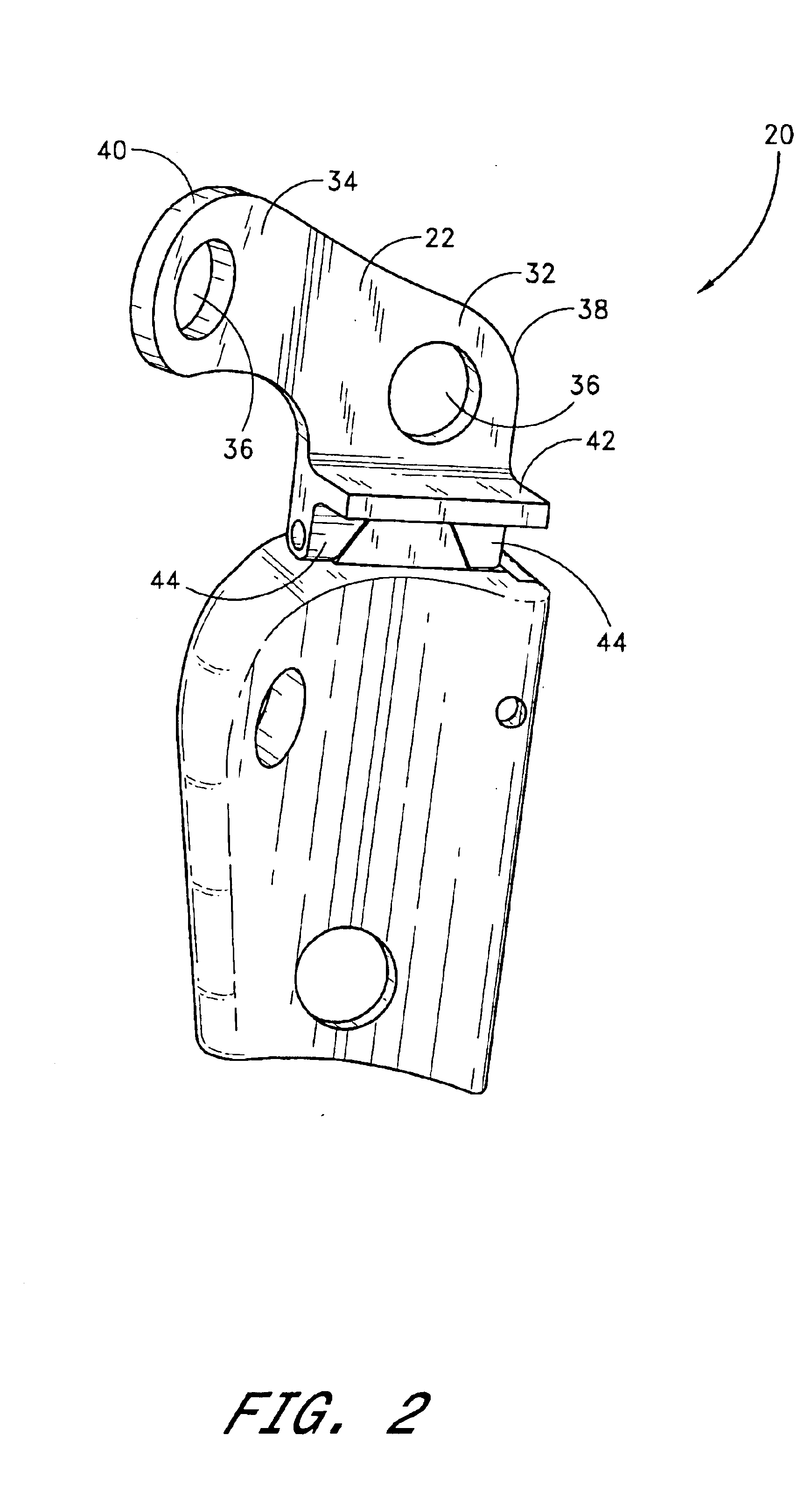
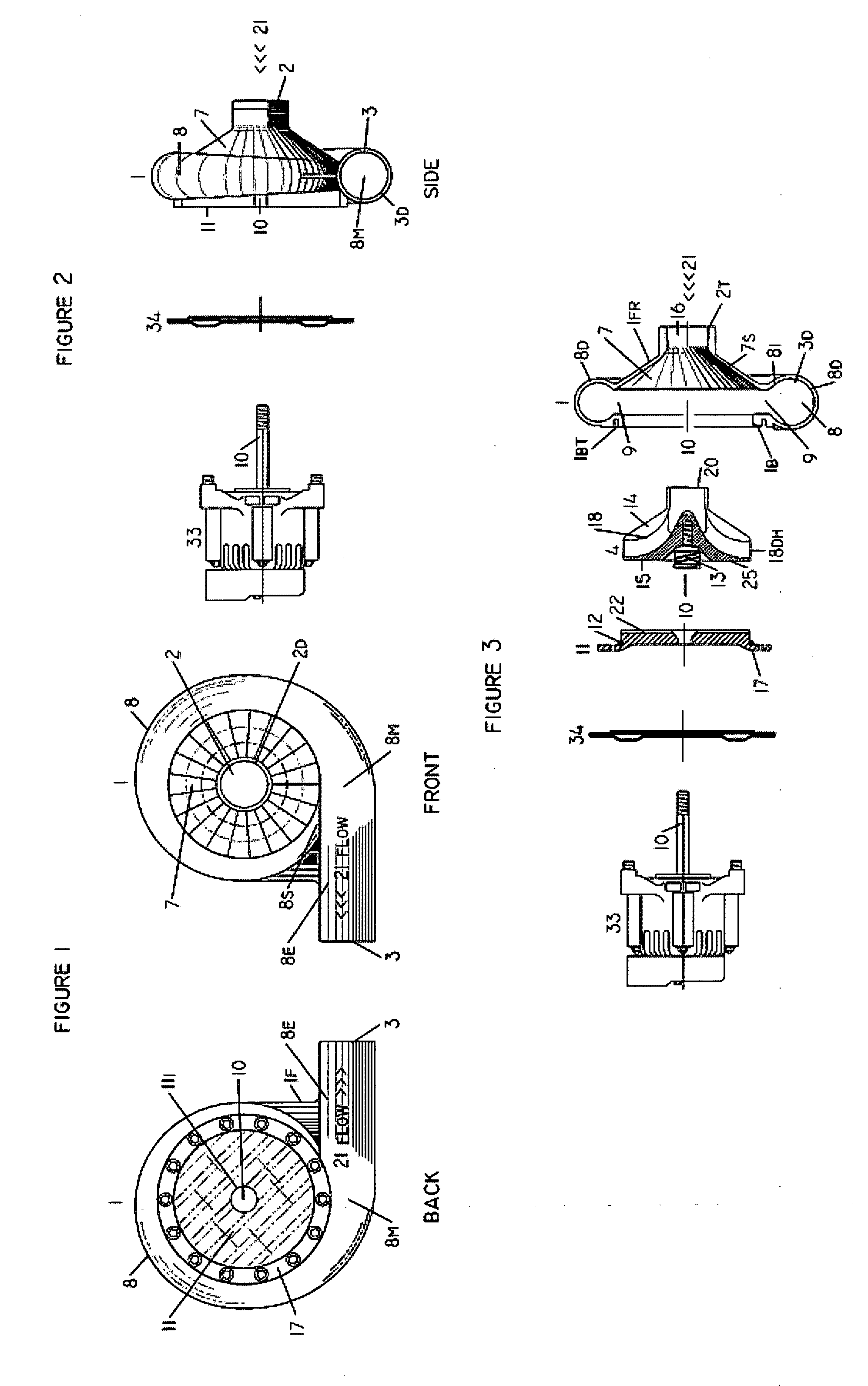
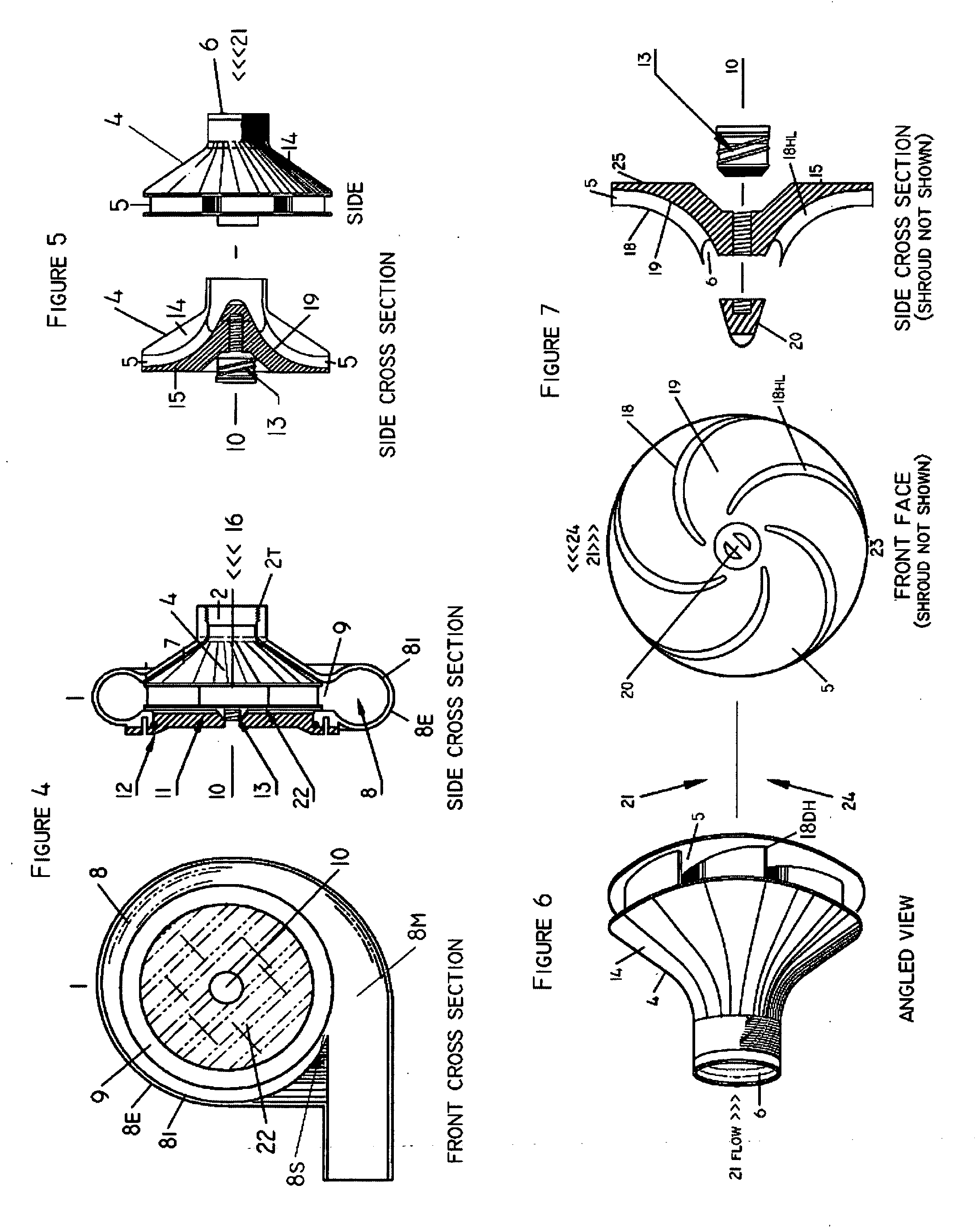
Internal osteotomy fixation device
InactiveUS6852113B2Flexibility in initially fittingLow profileInternal osteosythesisJoint implantsImplanted deviceConstant stress
An internal osteotomy fixation apparatus is provided. The device comprises a distal plate including a channel. A slide is slidably received within the channel such that the slide is translatable with respect to the distal plate along a first axis. A proximal plate is hingedly connected to the slide, such that the proximal plate has two degrees of freedom relative to the distal plate. A ledge protruding from a first portion of the proximal plate is configured to support a proximal bone segment when the device is implanted. The slide includes ratchet teeth. A ratchet arm including teeth is attached to the distal plate, and configured to engage the slide ratchet teeth. A cross-section of the ratchet arm is configured to maintain a constant stress level along a flexed portion of the arm. The distal plate includes a shelf upon which the ratchet arm rests. Compressive loads borne by the device are translated through the shelf to the distal plate. A minimum length of the device is related to the longer of the ratchet arm or the segment of teeth on the slide. The distal plate includes a hole through which a release mechanism is accessible.
Owner:ORTHOPAEDIC DESIGNS
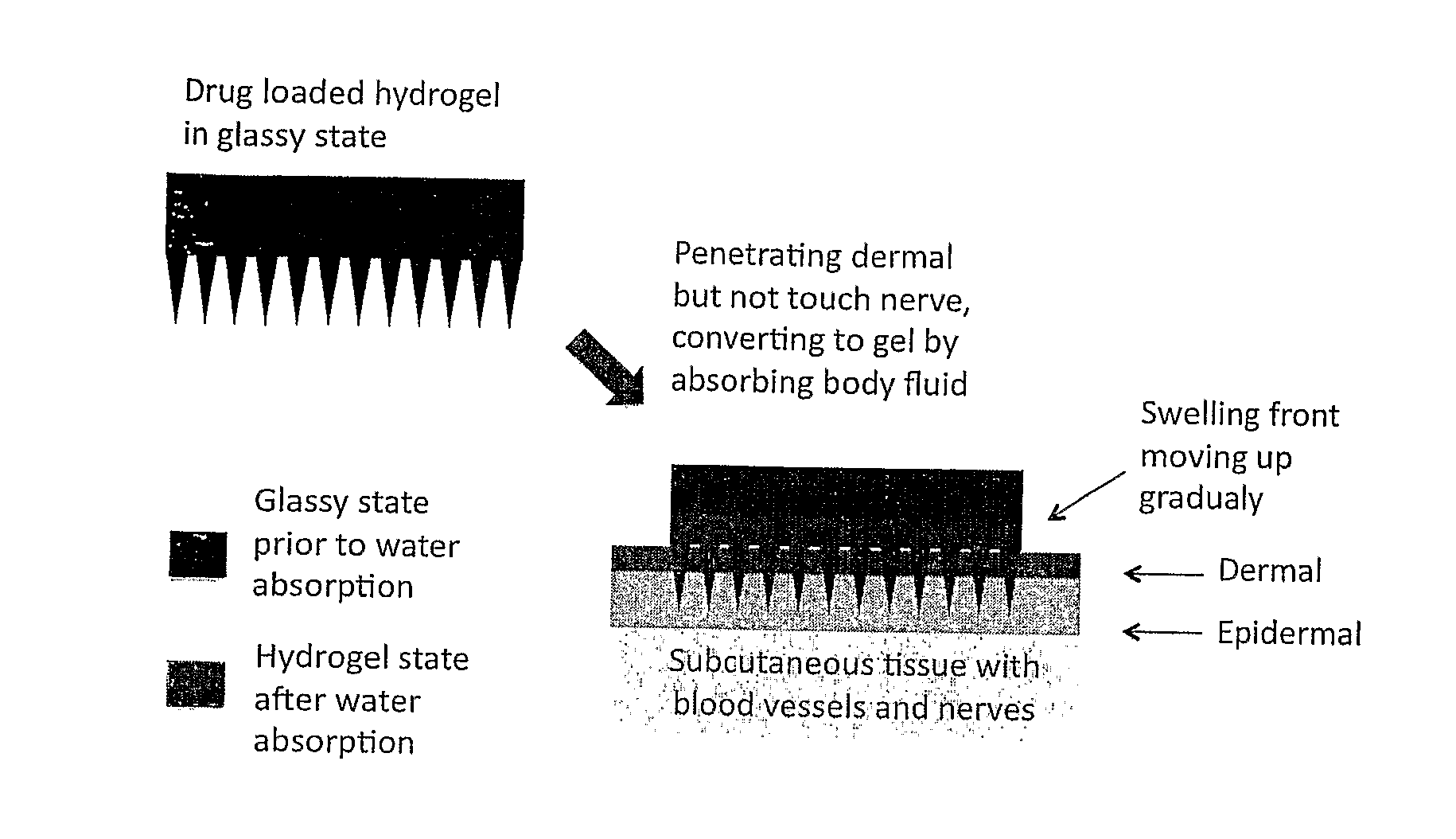
Phase-transition polymeric microneedles
ActiveUS20110195124A1Easy yet multi-functional fabrication processPowder deliveryPeptide/protein ingredientsOrganic solventMicrofabrication
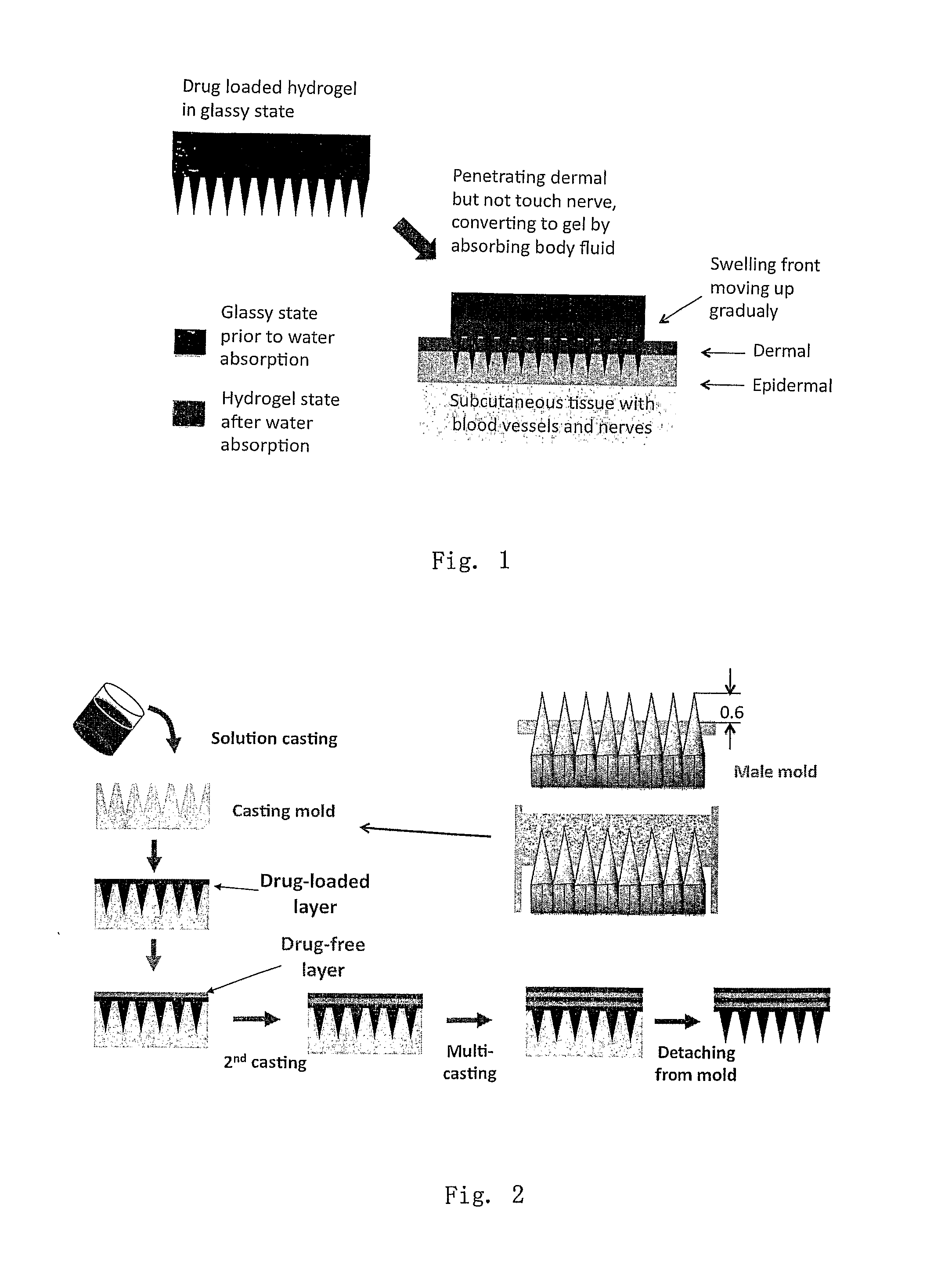
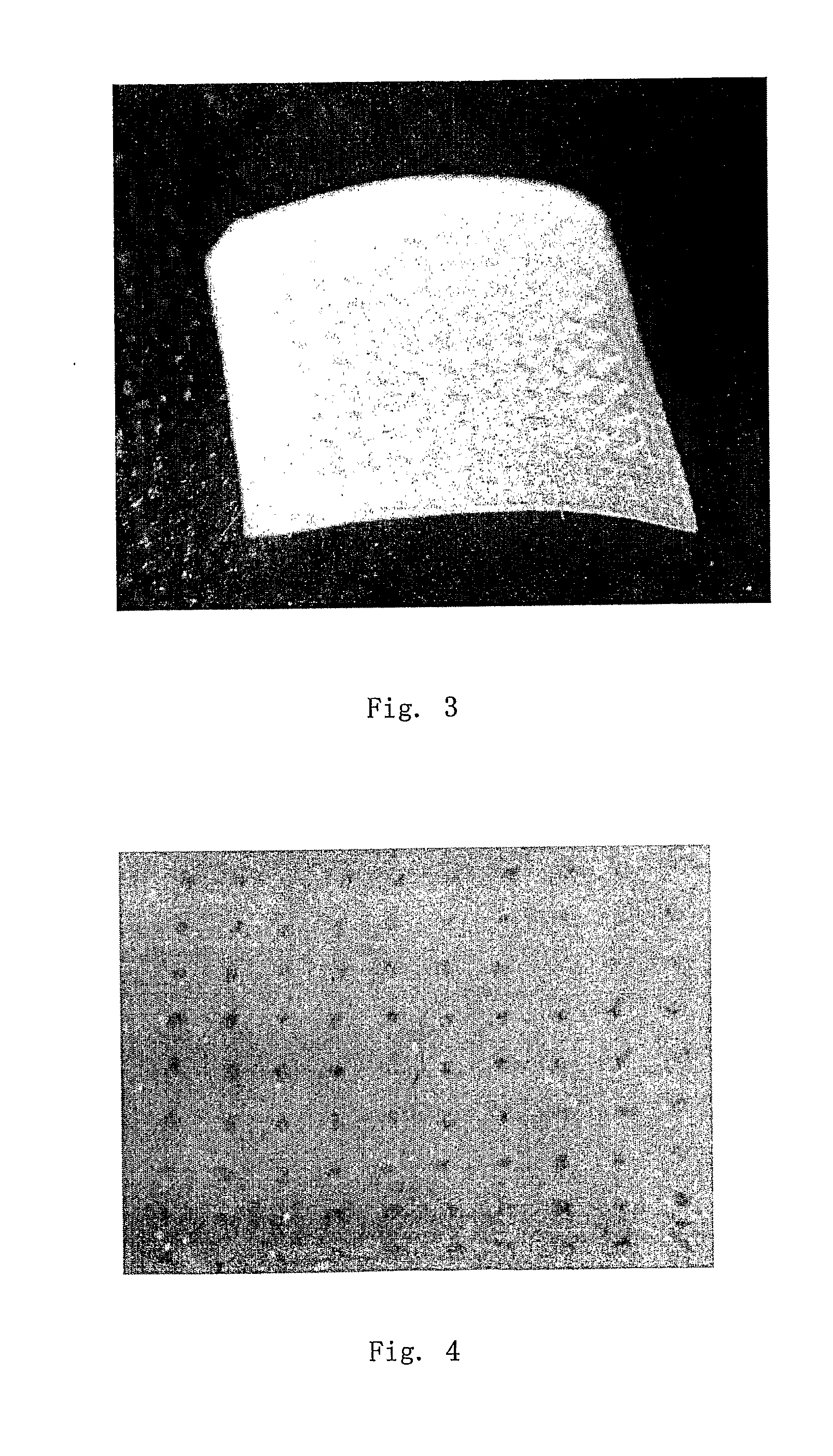
This invention discloses a novel microneedle system, phase-transition microneedle patch, which overcomes all the limitations that existing microneedles encountered. The microneedle patch is formed of an integrated polymeric piece consisting of a microneedle array and a plate (called holding plate) on which the needles stand. The microneedles of the patch are hard and strong enough to penetrate epidermis at dry state but turn to be hydrogel state soft and permeable to hydrophilic agents when absorbing body fluid. The hydrogel state of the patch is a hydrophilic network held by physical or chemical cross-linking junctions. The pores of the network are opened up by body fluid for drugs and macromolecules to diffuse through. The polymeric materials used to form the microneedle patch have been used in the pharmaceutical field for years and have proven compatibility with the skin and with proteins. The drugs may be stored in the matrix of the microneedle array as well as the holding plate so that the requirement for high dose applications may be full filled. In addition, molding (casting) of this type of microneedle patch is simple, easy to achieve and needs no microfabrication systems and organic solvents. By a programmed molding (casting), the patch may be assembled in a layered structure with desired drug concentration in each layer, respectively. Due to this design, a programmed pulse or a zero order release of drugs may easily be achieved. In addition, delicate proteins loaded in the patch are kept in a dry and hydrophilic glassy state before being released, the most favored state for protein storage. Finally, during the swelling-based drug release, the microneedle patch increases their thickness gradually between the skin and the back cover (which holds the needles) lo create a sustained pressure to ensure good contact of the microneedles inside epidermis.
Owner:JIN TUO
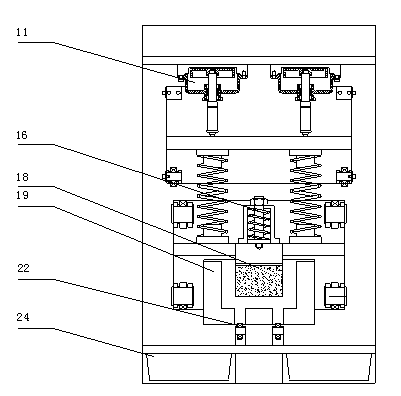
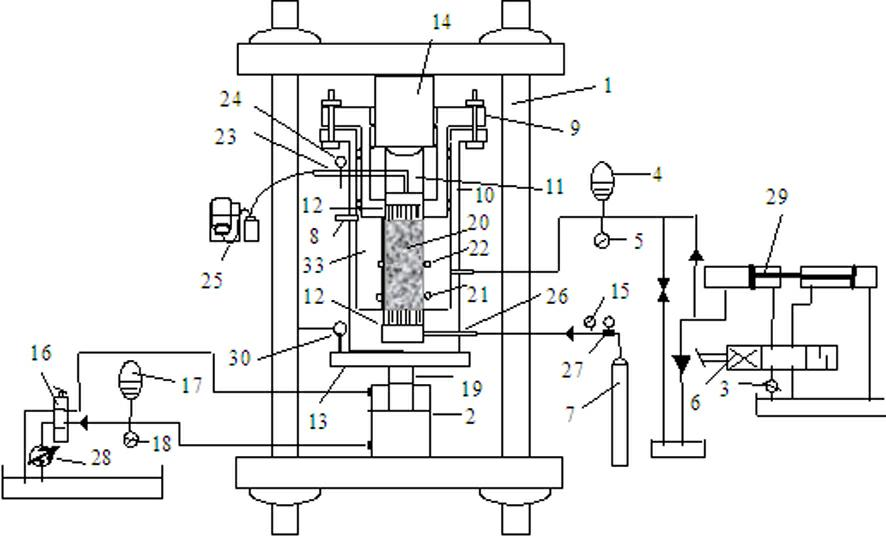
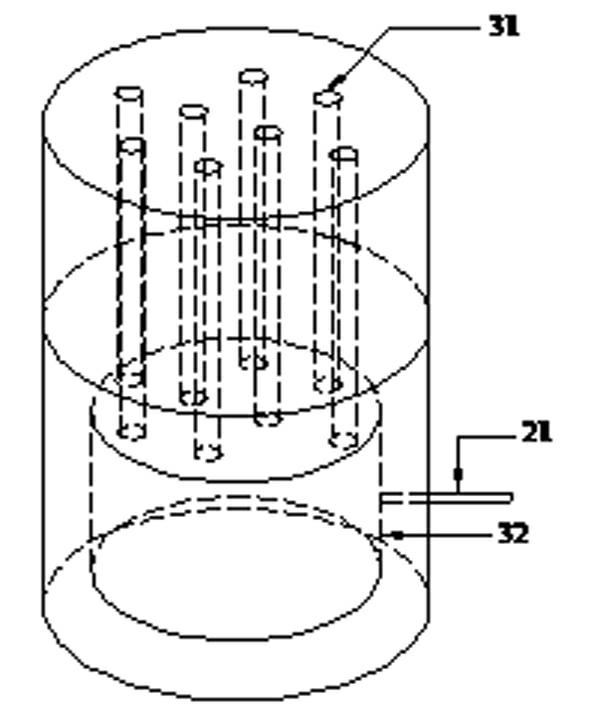
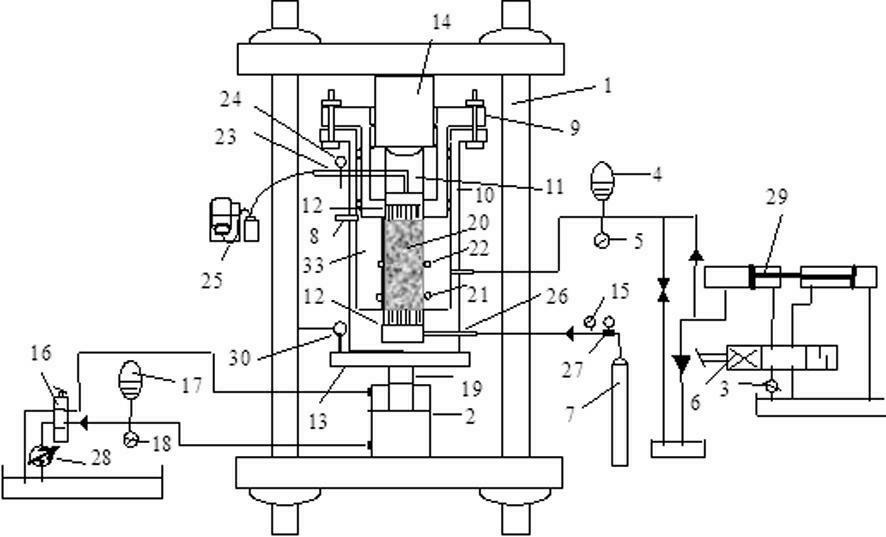
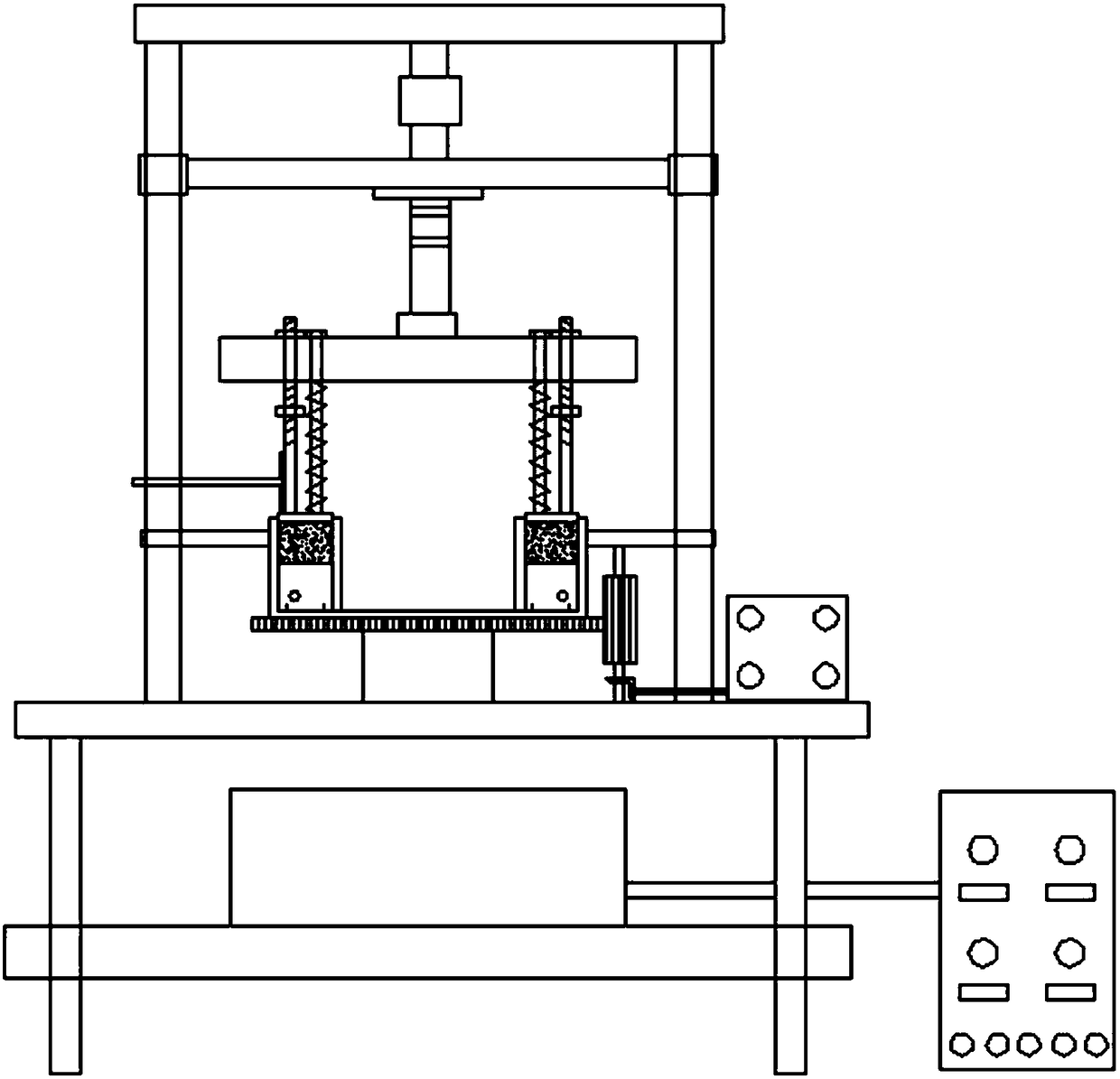
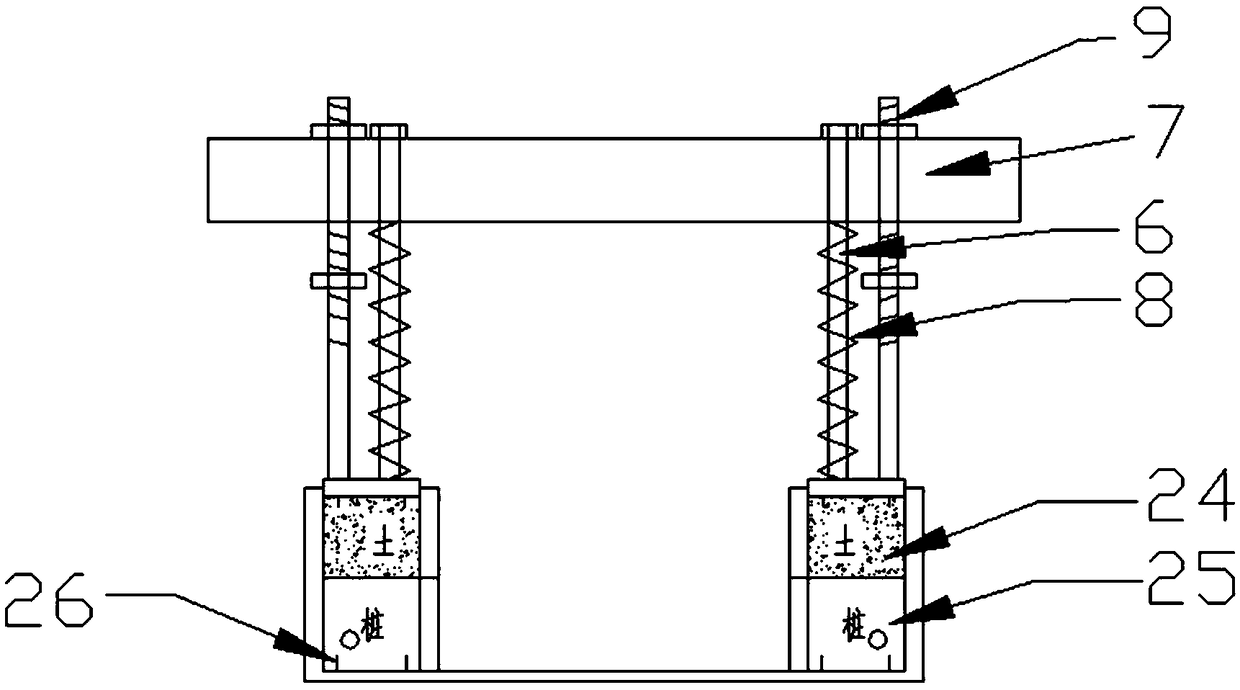
Triaxial test apparatus of soil under water-soil chemical action and method thereof
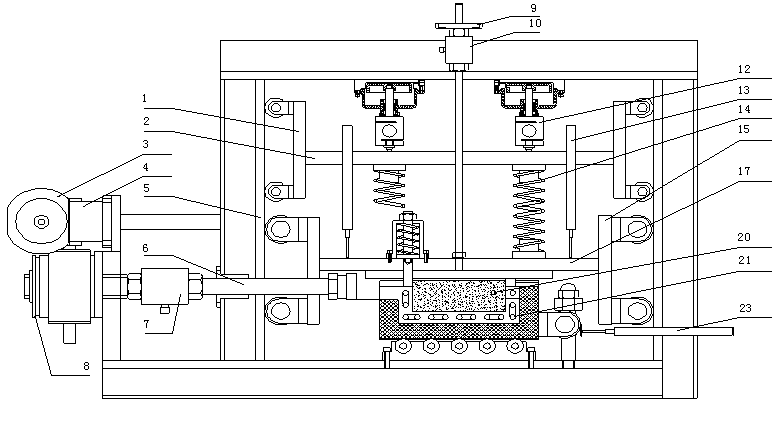
InactiveCN103091173ASolve the problem of accurate measurementPromote in-depth developmentMaterial strength using tensile/compressive forcesChemical solutionTriaxial shear test
The invention discloses a triaxial test apparatus of soil under water-soil chemical action and a method thereof, and relates to a testing technique of soil under a special environment. The device comprises a device frame (10), a triaxial pressure chamber (20), a confining pressure exerting and deformation test device (30), a solution cyclic displacement device (40), a stress / strain control loading device (50), a displacement sensor (60) and a data collecting and processing device (70). The uniformity of ingredients and concentration of internal pore water solution of a specimen is ensured by solution circulation; the change rules of soil deformation and strength caused by chemical solution transfusion under constant stress can be simulated and researched; the confining pressure exerting principle is improved; accurate measurement of body deformation is achieved; and the conversion of strain control and stress control can be achieved.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Large-scale multifunctional frozen soil-composition contact surface cycle direct shear apparatus and test operation method
ActiveCN102798575AEasy to controlPrecision stressMaterial strength using steady shearing forcesTemperature controlConstant stress
The invention is a large multifunctional frozen soil-structure interface cyclic direct shear apparatus and a test operation method. The structure of the apparatus comprises a horizontal and vertical loading system, a horizontal and vertical displacement and a load sensor, a temperature control and refrigeration system, a temperature sensor, a data collecting and self-stabilizing system, a guide rail system, and a soil sample box and supports. The test operation method comprises the steps of: 1. Installing the interface steel of required roughness; 2. Applying normal boundary conditions to a soil sample; 3. Cooling the soil sample; and 4. When the temperature cools to a target temperature, performing a shearing to the frozen soil interface in a selected shearing speed, at the same time the date collecting system beginning to record the stress-strain relation of the frozen soil interface. The apparatus and the method are the advantageous in that: 1) accurate control of the interface temperature in the temperature range between 0 DEG C and -20 DGE C can be achieved; 2) three normal boundary conditions of constant stiffness, constant displacement and constant stress can be accurately applied; 3) interfaces of various roughness can be simulated; 4) two shear modes of cyclic shear and monotonic shear can be realized; and 5) the stress applying and displacement control are accurate.
Owner:NANJING FORESTRY UNIV
Device for testing gas seepage and creepage coupling action of rocks
InactiveCN102494981AApply evenlyGood voltage stabilization effectInvestigating material ductilityPermeability/surface area analysisCouplingStress level
The invention provides a device for testing gas seepage and creepage coupling action of rocks. The device comprises an upper seat, a lower seat, a motor oil pump, a high-pressure nitrogen cylinder and an oil pump, wherein the upper seat is connected with the lower seat; a gas cavity with honeycomb-shaped gas holes is connected to each of the upper seat and the lower seat; the gas cavity which is provided with the honeycomb-shaped gas holes and connected with the upper seat is connected with an outlet pipe; the gas cavity which is provided with the honeycomb-shaped gas holes and connected with the lower seat is connected with a gas inlet pipe; the lower seat and the gas cavity which is provided with the honeycomb-shaped gas holes and connected with the lower seat are connected with a carrying plate; the carrying plate is connected with a loading oil cylinder through a pressurization piston rod; the motor oil pump is connected with the loading oil cylinder; the high-pressure nitrogen cylinder is connected with the gas inlet pipe; and the oil pump is connected with the oil cylinder and a triaxial cavity. The device can be used for performing a gas seepage and creepage coupling test on the rocks under different stress levels and different gas seepage pressures, so that the creepage test for a rock sample can be finished successfully under constant stress; and data is real and reliable.
Owner:HUNAN UNIV OF SCI & TECH
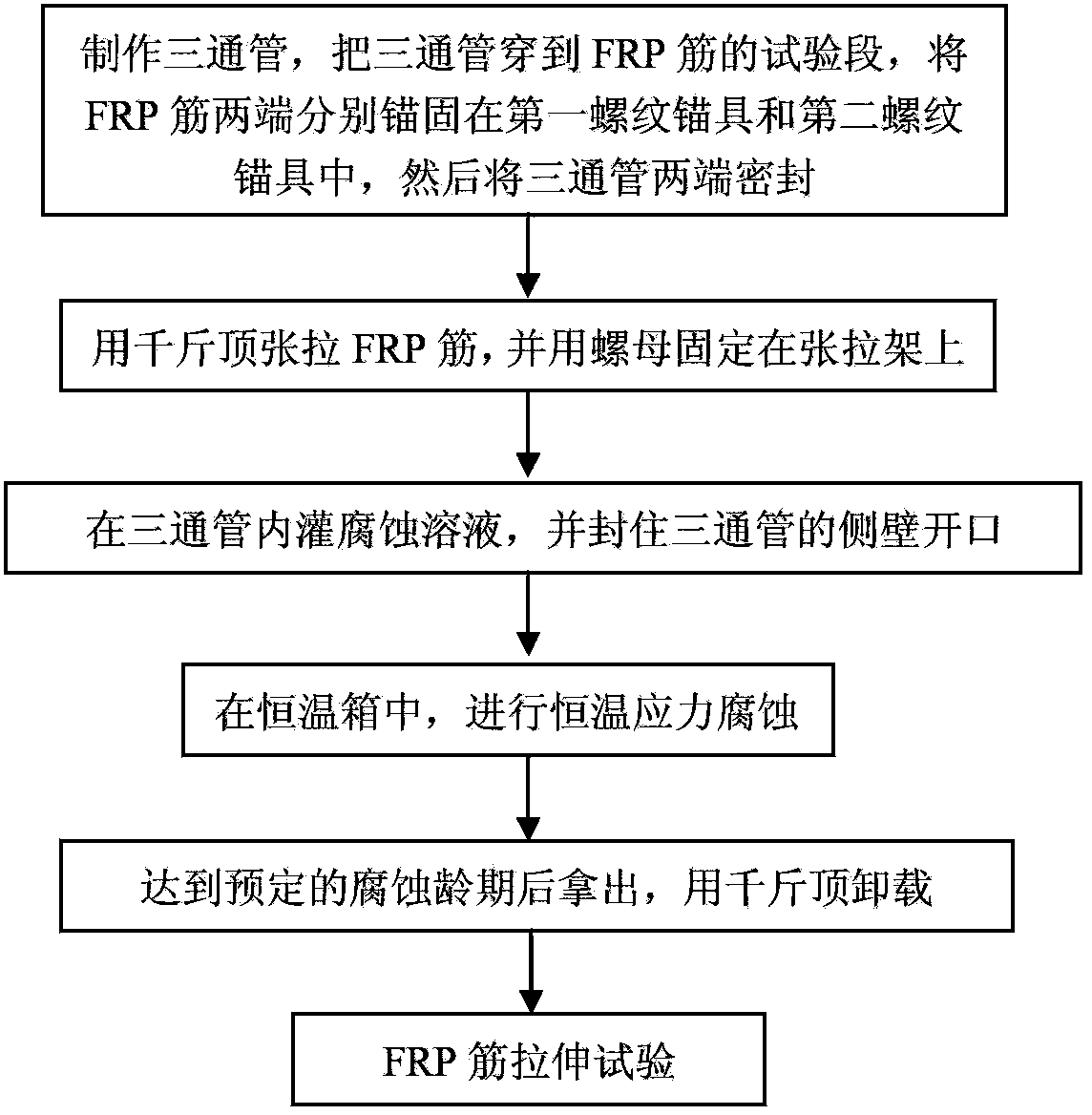
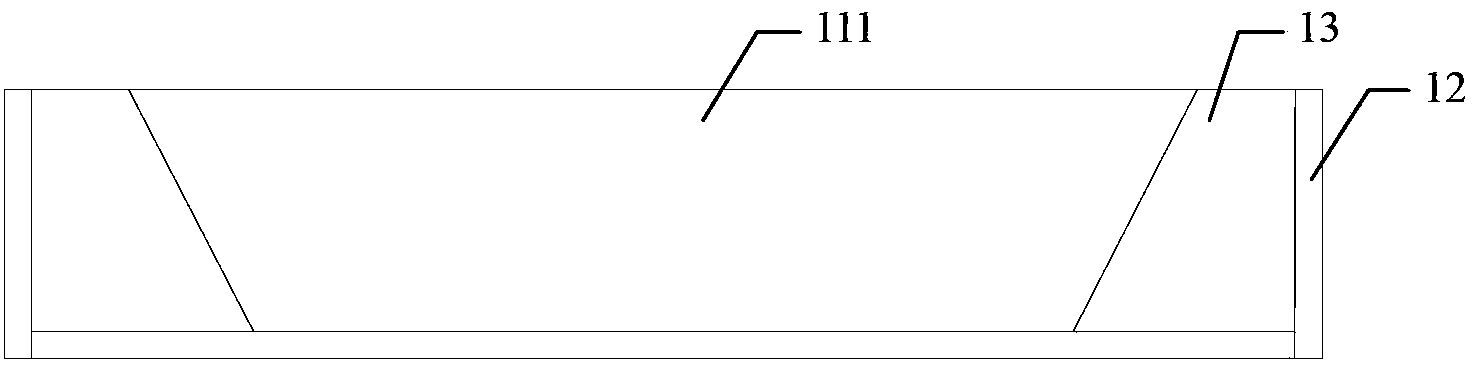
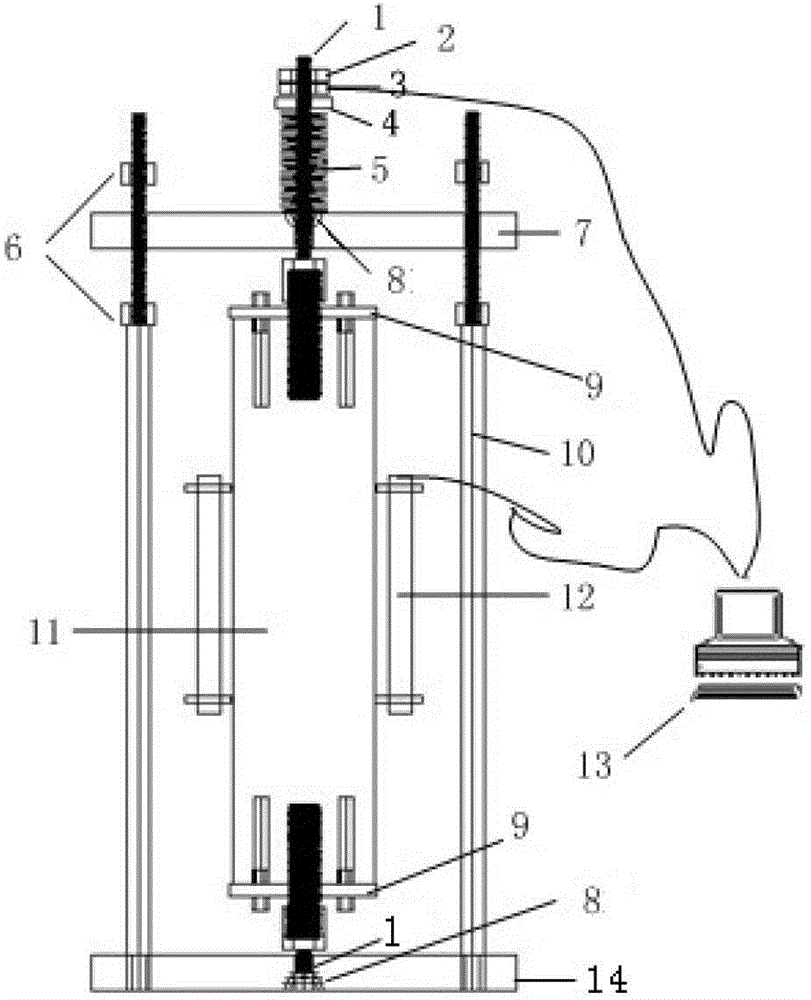
Constant-temperature stress corrosion testing device and method for FRP (fiber reinforced polymer) tendon
InactiveCN103376222ASimple structureEasy to manufactureMaterial strength using tensile/compressive forcesTemperature stressFiber
The invention discloses a constant-temperature stress corrosion testing device and method for an FRP (fiber reinforced polymer) tendon. The device comprises a stretch-draw frame, threaded anchors, a three-way pipe, a spring, a counter force frame, a lifting jack, a force sensor and a stretch-draw anchor rod. Certain stretch-draw stress is applied to the FRP tendon by utilizing the stretch-draw frame, the counter force frame, the lifting jack and the force sensor, and the FRP tendon is fixed by a nut, and the stress loss is compensated by the compression spring, and the constant stress level can be kept for a long time. A corrosion solution soaking environment is provided through a method that a corrosion solution is filled into a home-made PVC pipe device fixed in the middle of the FRP tendon, so that the FRP tendon is in the corrosion solution soaking environment for a long time. After the corrosion solution is filled completely and the prestress is stretched and drawn, the whole stretch-draw frame for stretching and drawing the FRP tendon is put into a constant-temperature constant-humidity test box so as to guarantee the long-term constant-temperature environment. After the constant-temperature corrosion, the device is unloaded through the steps the same with the stretch-draw step, the FRP tendon is taken down for a stretch test. The method comprises the following steps of: anchoring the FRP tendon, fixing through stretching and drawing, corroding at constant temperature, unloading and carrying out the stretch-draw test.
Owner:SOUTHEAST UNIV
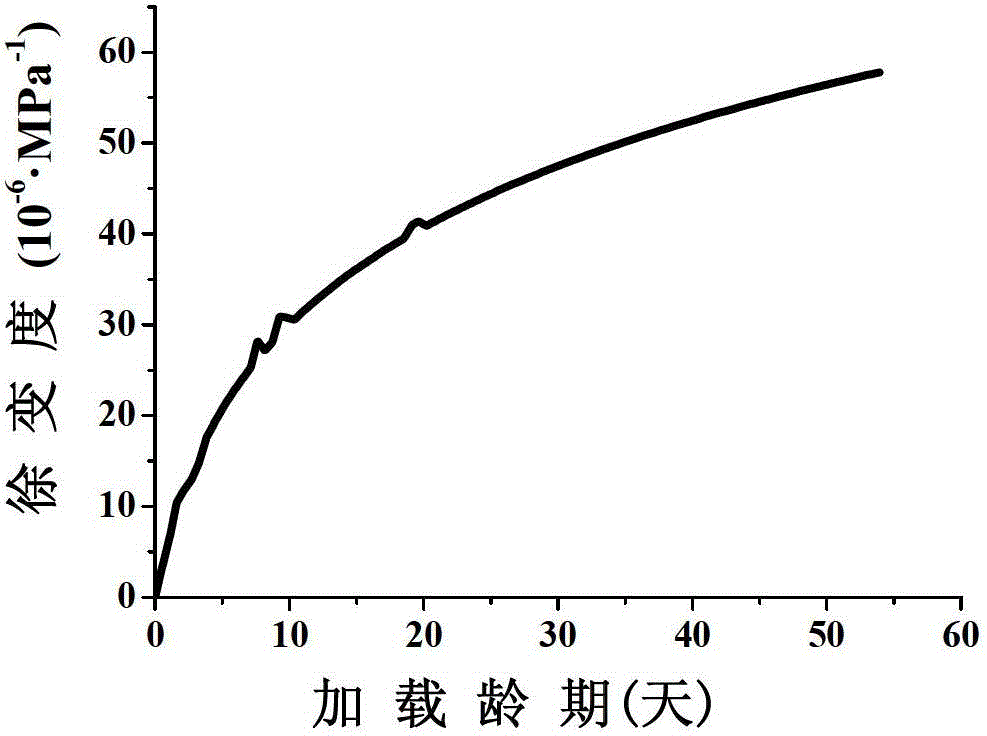
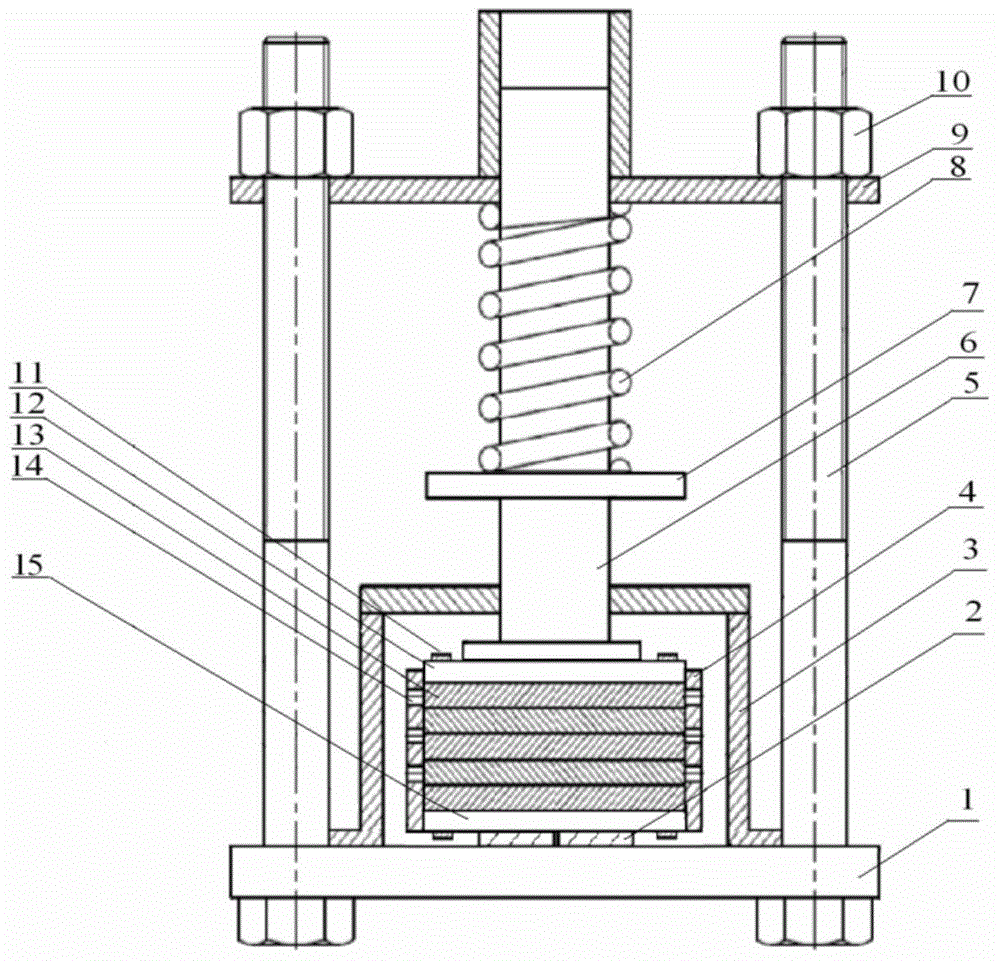
Concrete axis stretching creep tester and test method thereof
InactiveCN103149100AImprove continuityRealize acquisitionInvestigating material ductilityStress concentrationConstant stress
The invention discloses a concrete axis stretching creep tester and a test method thereof. The tester comprises an upper pulling plate, a lower pulling plate, pulling bars, screws, a spring, a stress sensor and vibrating string extensometers, the pulling bars are fixed on the lower pulling plate, and the upper ends of the pulling bars are provided with thread ends; the upper pulling plate traverses the upper ends of the pulling bars and is sleeved on the thread ends, and the thread ends at the upper and lower sides of the upper pulling plate are provided with nuts to fix the upper pulling plate; the upper and lower pulling plates are provided with the screws respectively and are used for the fixed connection of the upper and lower end of a concrete test piece, and the vibrating string extensometers are arranged at two sides of the concrete test piece; and the screw on the upper pulling plate stretches out of the upper pulling plate, the spring is sleeved on the stretching end of the screw, the stretching screw is also sequentially sleeved with a pad and a nut, and the stress sensor is arranged between the pad and the nut. The tester and the method realize the continuous automatic detection and acquisition of deformation; and no eccentricity, concentrated stress and constant stress are realized in the stretching process, so the test accuracy is improved.
Owner:SOUTHEAST UNIV
Predicting method of large-size NEPE propellant loading storage life
The invention discloses a predicting method of large-size NEPE propellant loading storage life, which includes following steps: (1) performing constant-stress load single-temperature acceleration test, which includes operations of making an NEPE propellant acceleration aging test sample under constant-stress load and performing a single-temperature-stress level temperature acceleration life test; (2) researching the performance change law during an NEPE propellant loading aging process; (3) determining a failure model of the NEPE propellant loading; and (4) carrying out acceleration life tests at different temperatures and under different pressures to predict the large-size NEPE propellant loading storage life. In the method, by means of loading pressure to simulate the self weight on a large-size NEPE propellant during storage, the performance change law of the NEPE propellant under a temperature-pressure double-stress effect. In addition, with tensile strength as a failure parameter, the method of evaluating the NEPE propellant loading storage life is disclosed.
Owner:XIAN MODERN CHEM RES INST
Designing and prediction method for converting high-temperature stress relaxation data into creep data
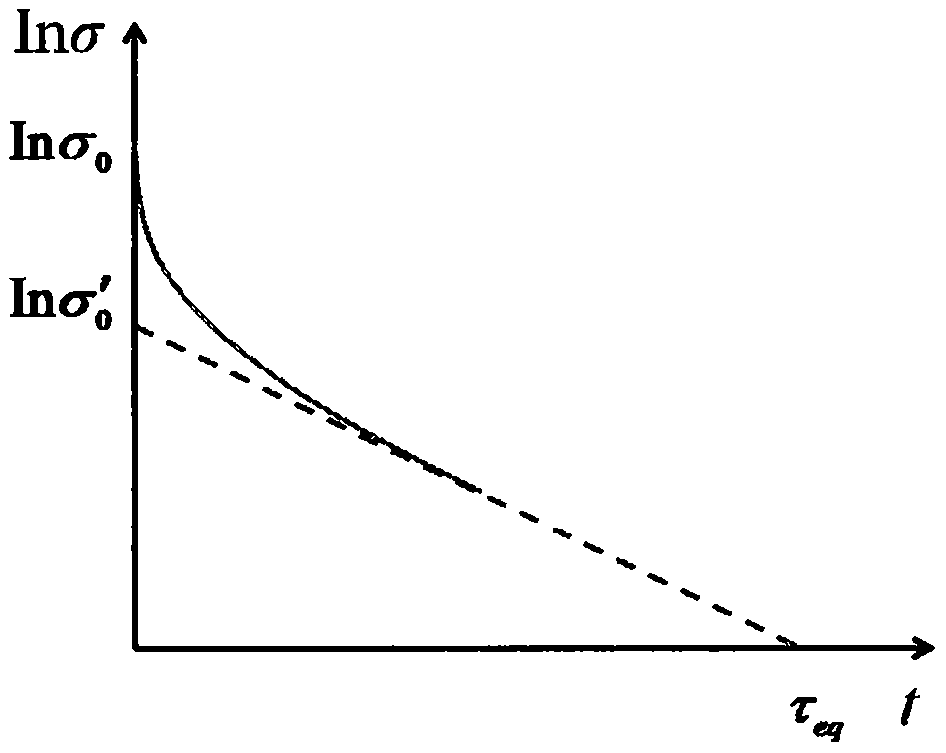
InactiveCN105842087ALow conversion accuracyReasonable technical pathInvestigating material ductilityComplex mathematical operationsTemperature stressConstant stress
The invention discloses a designing and prediction method for converting high-temperature stress relaxation data into creep data. The method mainly comprises the following steps: establishing a stress relaxation two-stage superposition model; creating the concepts of equivalent full relaxation time and equivalent relaxation creep rates, carrying out simulation of an equivalent relaxation creep rate in two integrated relaxation stages to obtain a steady-state creep rate under constant stress and establishing a stress relaxation-creep conversion model; acquiring stress relaxation performance data of a high-temperature component material; subjecting the data and the stress relaxation two-stage superposition model to fitting so as to obtain a corresponding material constant; and carrying out calculating according to a numerical analysis program, solving the stress relaxation-creep conversion model and eventually converting stress relaxation data into creep data so as to obtain a steady-state creep rate curve and a steady-state creep curve. According to the invention, accurate association between stress relaxation data and creep data behaviors is established; a prediction method for converting single relaxation data into creep data under any stress is constructed; so a novel approach is provided for designing of creep strength of a high-temperature member and for evaluation of the service life of the high-temperature member.
Owner:ANYANG INST OF TECH
High-temperature high-pressure constant load stress corrosion experiment method and device
InactiveCN102706750ARealistic simulation of mechanical damageStability test methodWeather/light/corrosion resistanceMaterial strength using tensile/compressive forcesMetallic materialsStressed state
The invention discloses a high-temperature high-pressure constant load stress corrosion experiment method and device. The technical scheme are that constant load stress corrosion experiment of metal materials can be conducted in certain stress state under the combined action of high-temperature high-pressure gaseous phase and liquid phase corrosion media, and subjected to combined action of the constant stress, and high-temperature high-pressure gaseous phase and liquid phase corrosion media in experiment process, and evaluation and research on the applicability and action mechanism of the metal material can be conducted under high-temperature high-pressure corrosive environment and tension stress state according to the experiment results. Damage to strength, plasticity and flexibility of the metal materials can be determined in high-temperature high-pressure gaseous phase and liquid phase corrosive environments under the action of any load by utilizing the experiment method and device, the mechanical property damage degree of the metal materials can be obtained in certain stress state and special corrosion environment according to the test result, thus conducting preference of the metal materials and applicability evaluation.
Owner:SOUTHWEST PETROLEUM UNIV
Statistical analysis method of accelerated life tests based on fuzzy theory
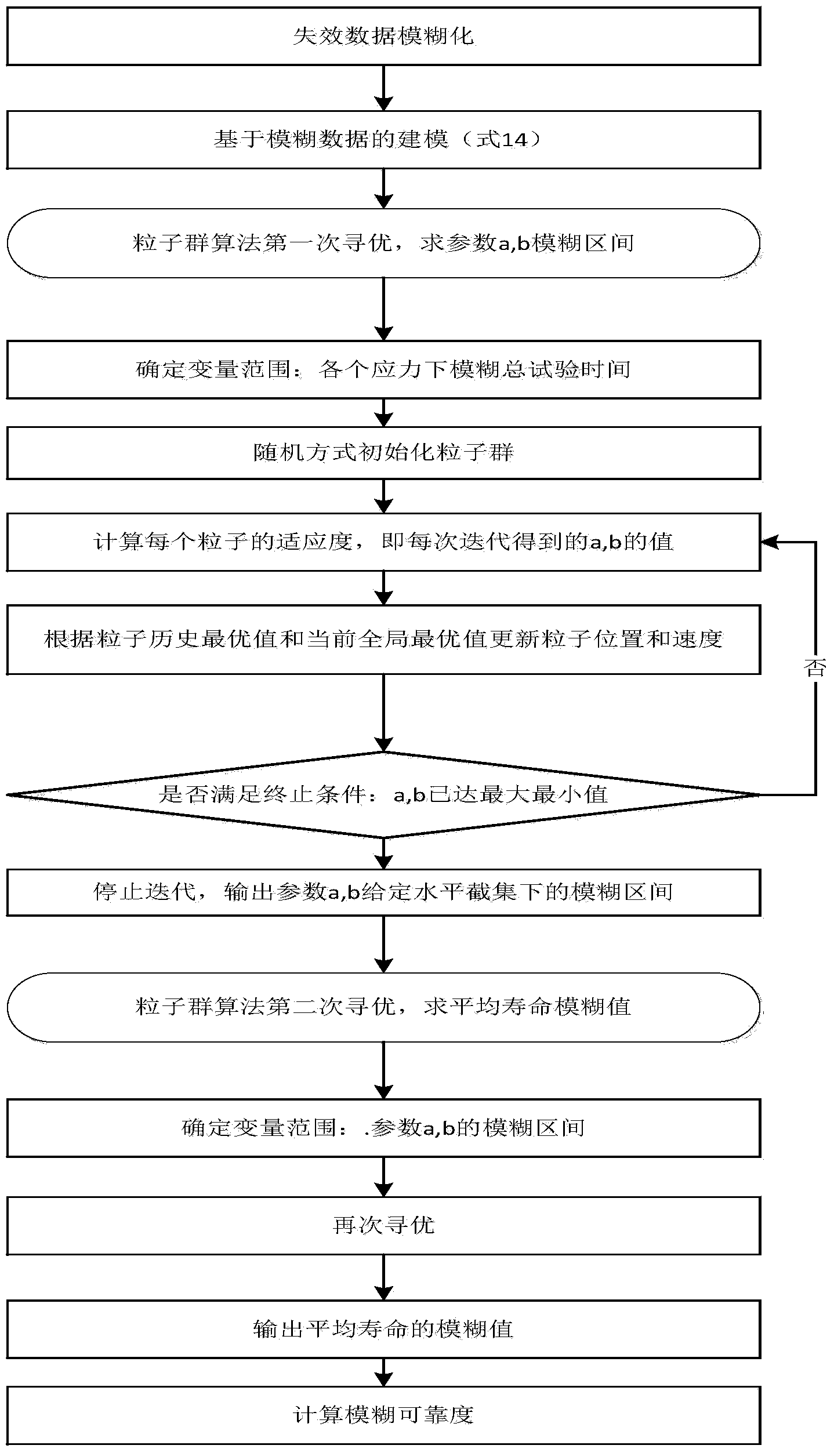
ActiveCN104077445AThe result is reasonableHas reference valueSpecial data processing applicationsPrediction intervalConstant stress
The invention discloses a statistical analysis method of accelerated life tests based on a fuzzy theory. The statistical analysis method comprises a first step of reasonably fuzzifying failure terminated censoring of constant-stress accelerated life tests, thereby obtaining fuzzy failure data, a second step of building a fuzzy statistical model of the accelerated life tests by use of a maximum likelihood method, and a third step of performing estimation on model parameters and fuzzy prediction on lifetime and reliability. The statistical analysis method is capable of providing the fuzzy estimation values of the model parameters according to the built model and further providing the fuzzy lifetime prediction interval and the fuzzy reliability interval of products; compared with point estimation values provided by a traditional statistical analysis method, the results of the statistical analysis method are more reasonable and have better reference value.
Owner:BEIHANG UNIV
Method for describing creep curve of metal material
ActiveCN105004617ASimple structureHigher than precisionMaterial strength using tensile/compressive forcesConstant stressMetallic materials
Owner:SHENYANG POLYTECHNIC UNIV
Method for electrically conductively connecting a contact piece to an electrical conductor, and corresponding arrangement
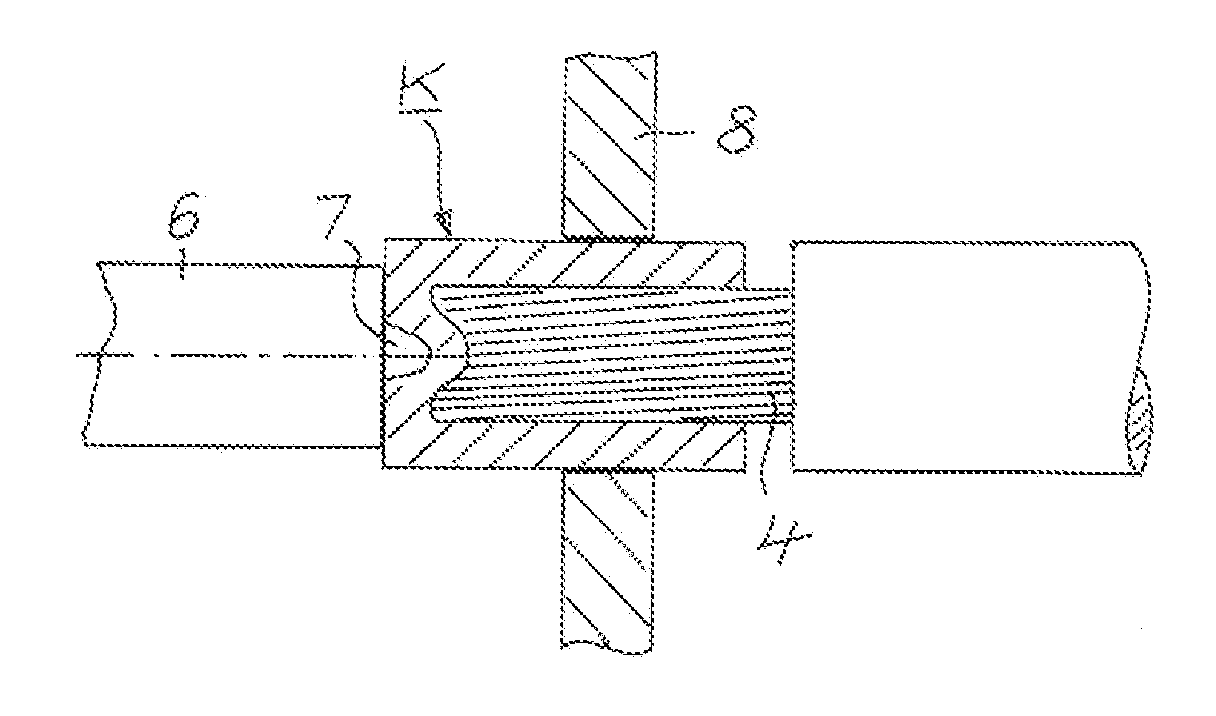
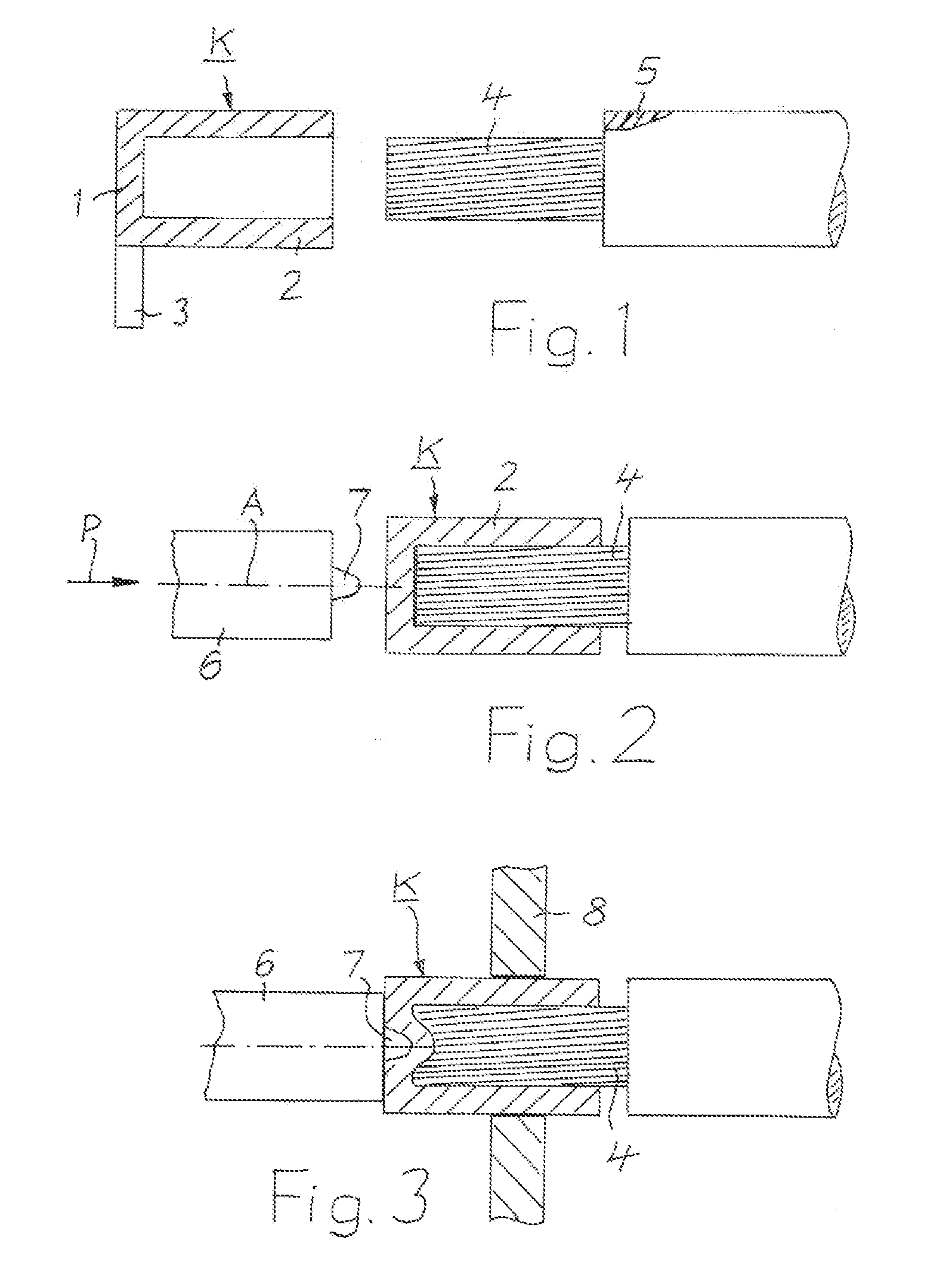
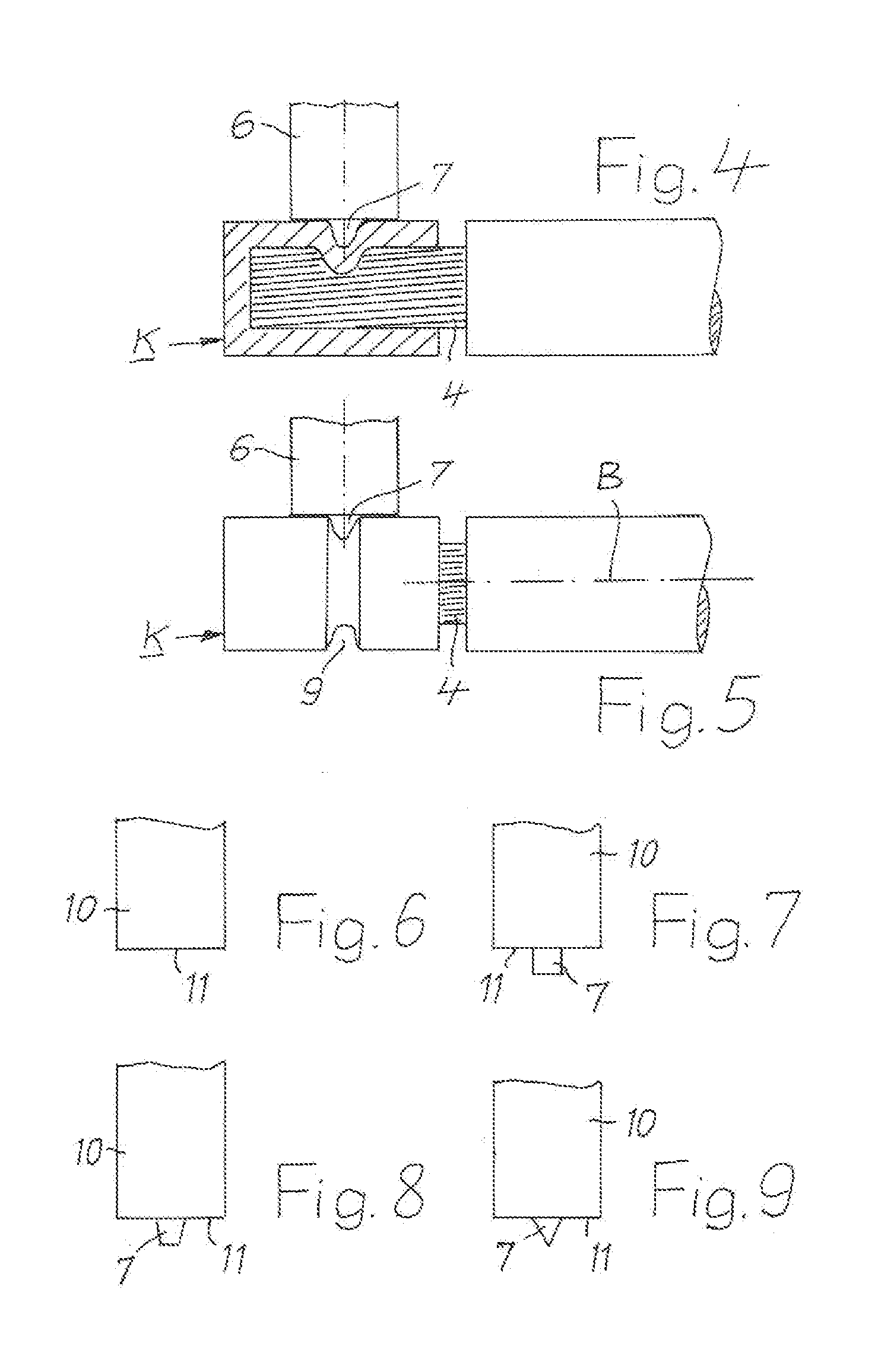
InactiveUS20140144015A1Active connectionContact member assembly/disassemblySoldered/welded connectionsElectrical conductorConstant stress
A method is provided for electrically conductively connecting a contact part on the basis of copper to an electrical conductor composed of a plurality of individual wires containing aluminum. A cup-shaped contact part which has a bottom and a cylindrical sleeve, which is integrally connected to the bottom and protrudes from the bottom, is pushed with tight contact of the sleeve against the conductor to such an extent until the end face of the conductor rests against the bottom of the contact part. Consequently, at least one rotating tool (6) is applied with sustained pressure, until due to increased temperature of the conductor, the conductor is softened to such an extent that it integrally connects with the contact part. The wall of the contact part is not broken through by the tool. Finally, the tool is removed from the contact part.
Owner:NEXANS
Deterministic cleave of optical fiber
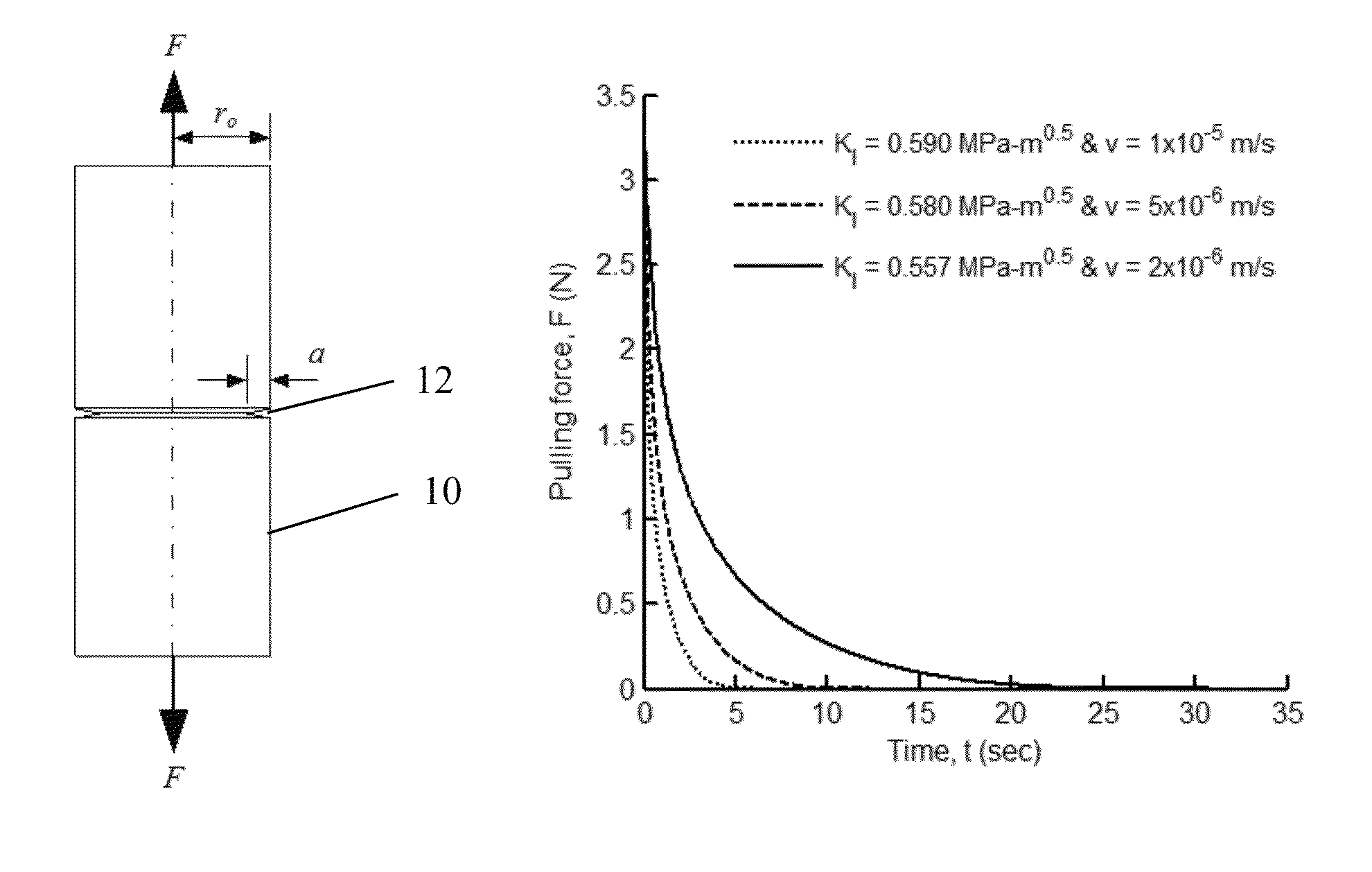
ActiveUS8740029B2Simply and reliably deployedEffective and efficient and reliable approachCoupling light guidesMetal working apparatusFiberStrain energy
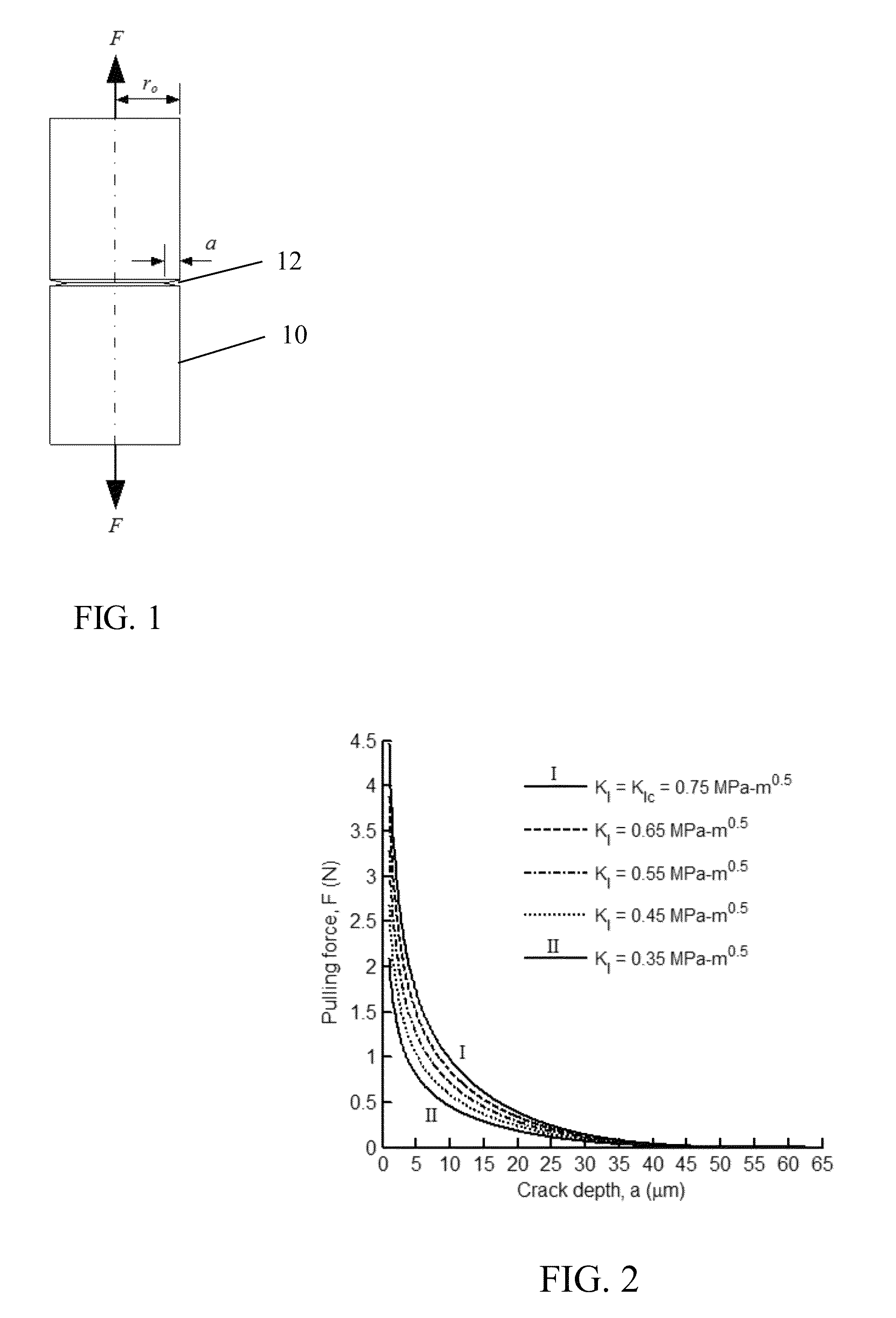
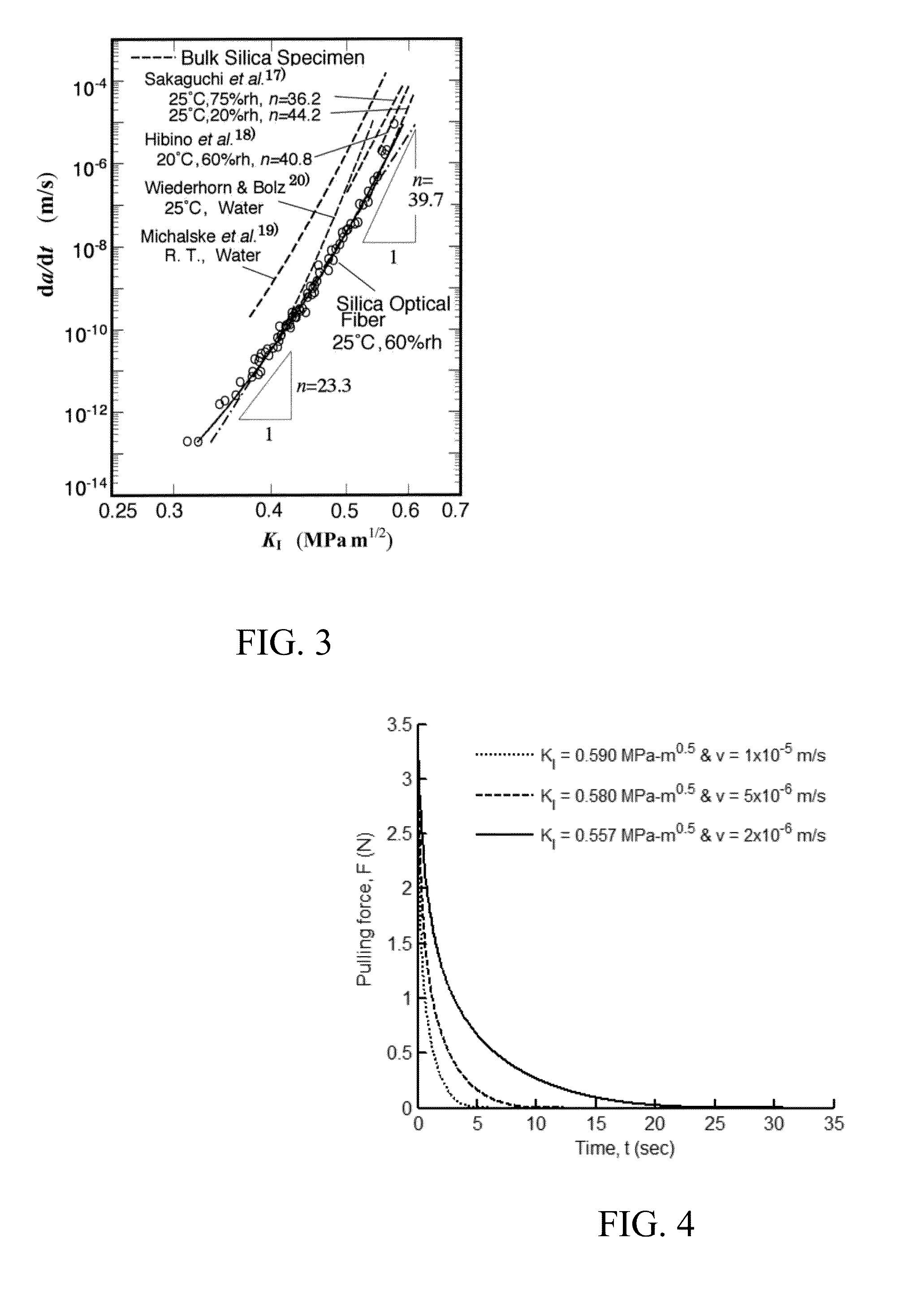
Axial tension is applied to an optical fiber that had been scored at the intended cleave location, wherein the axial tension is applied in a time-varying manner to maintain the stress intensity factor for crack on the fiber within an acceptable level to produce a stable crack growth at a reasonable rate to cleave the fiber without requiring polishing of the end surface. Careful control of the applied tension force with time acts to control the velocity of the propagating crack by maintaining substantially constant stress intensity factor. The applied axial tension force is reduced with time and / or crack growth (as crack propagates). As a result, the strain energy in the fiber material is released by formation of a single plane with an optical quality surface without requiring polishing. A substantially flat optical surface of enhanced optical quality is formed at the cleaved end of the optical fiber.
Owner:SENKO ADVANCED COMPONENTS
Concrete performance testing device under combined action of constant tensile load and erosion medium
ActiveCN104155185AReduce or eliminate eccentricityAddress stress relaxationWeather/light/corrosion resistanceMaterial strength using tensile/compressive forcesConstant stressEngineering
The invention discloses a concrete performance testing device under the combined action of a constant tensile load and an erosion medium, relates to the field of concrete durability testing and solves the problem that an existing testing device cannot implement concrete performance testing under the combined action of a constant stress and medium erosion. The testing device comprises a bottom plate, a connecting base, a first puller, a middle pressure plate, an upper pressure plate, a pull rod, a dish-shaped spring and a medium circulating device, wherein the bottom plate is fixedly connected with an upright; the connecting base is fixed in the middle of the bottom plate; the first puller is hinged to the connecting base and can rotate around the connecting base; the middle pressure plate passes through the upright and is fixed at a limiting point of the upright; the upper pressure plate passes through the upright; the pull rod passes through through holes in the middle pressure plate and the upper pressure plate; one end of the pull rod is connected with a spherical joint; a second puller is connected to the spherical joint; a nut is screwed at the other end of the pull rod; the dish-shaped spring sleeves the pull rod and is positioned between the middle pressure plate and the upper pressure plate; one end of a concrete test piece is connected to the first puller and the other end of the concrete test piece is connected to the second puller.
Owner:CHINA BUILDING MATERIALS ACAD
Radial balance mechanism for automatic wire arranging device of multi-wire cutting machine
ActiveCN102101325AGuaranteed uniformityGuaranteed forceWorking accessoriesFine working devicesWire cuttingConstant stress
The invention discloses a radial balance mechanism for an automatic wire arranging device of a multi-wire cutting machine, which is used for ensuring that cutting metal wires on a coiling and uncoiling wheel are arranged sequentially. The radial balance mechanism comprises a frame, wherein cutting metal wires (15) are guided and arranged on a coiling and uncoiling wheel (16) through a wire guide pulley (1) and a tension control pulley (9). The radial balance mechanism is characterized in that: the frame is provided with a slide block (4) which can move along the axial direction of the coiling and uncoiling wheel (16); the slide block (4) is provided with a swinging wire guide pulley (11) which can swing along the radial direction of the coiling and uncoiling wheel (16); and the cutting metal wires (15) are guided and arranged on the coiling and uncoiling wheel (16) through the wire guide pulley (1), the tension control pulley (9) and the swinging wire guide pulley (11). The radial balance mechanism has a simple structure, can automatically balance tension and direction during coiling and uncoiling and always keeps tangential with the coiling and uncoiling wheel and vertical coiling and uncoiling, so that the uniformity of wire arrangement and constant stress are ensured, a cutting effect is ensured and the quality of a cutting product is greatly enhanced.
Owner:HUNAN YUJING MACHINE
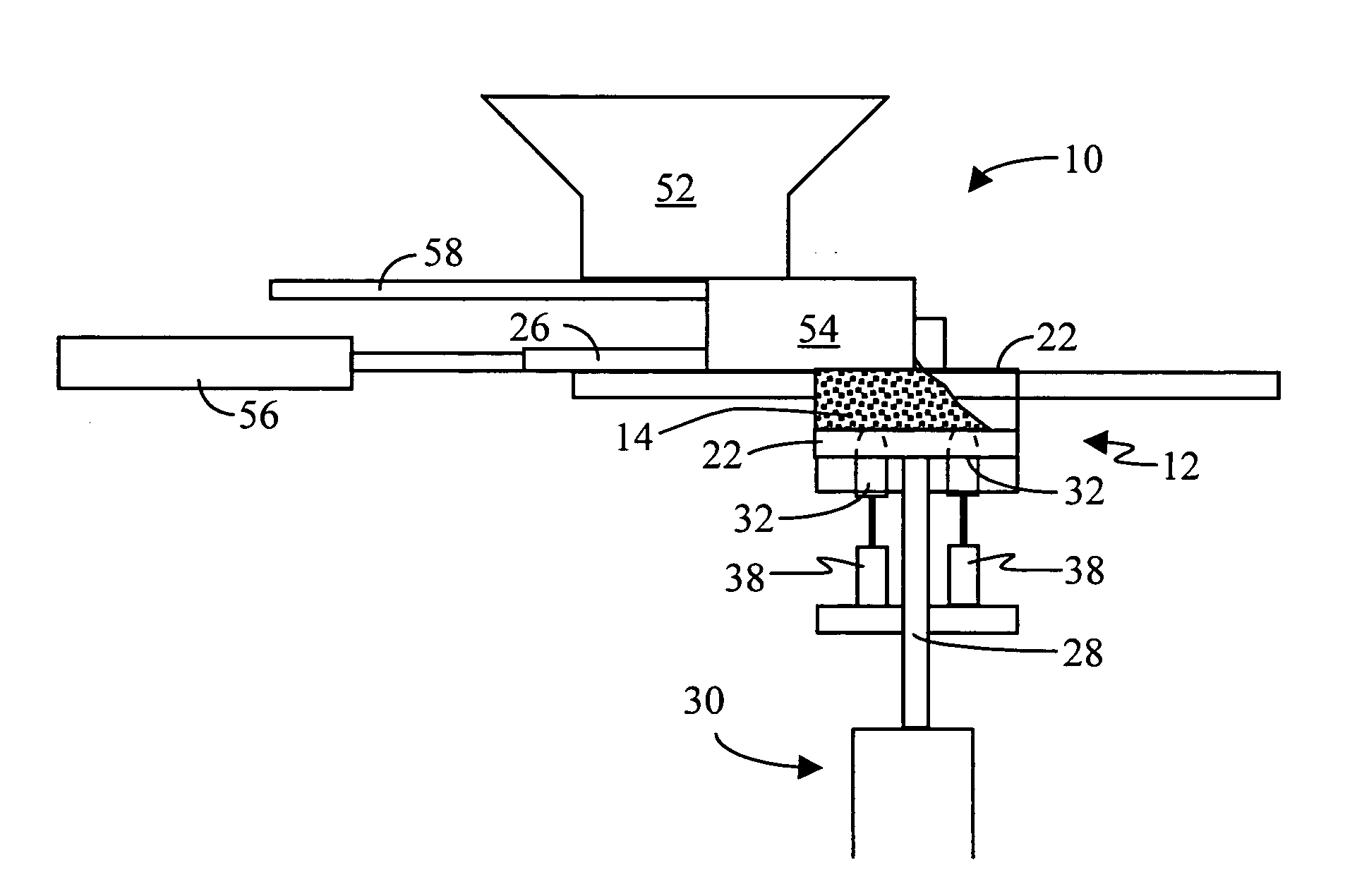
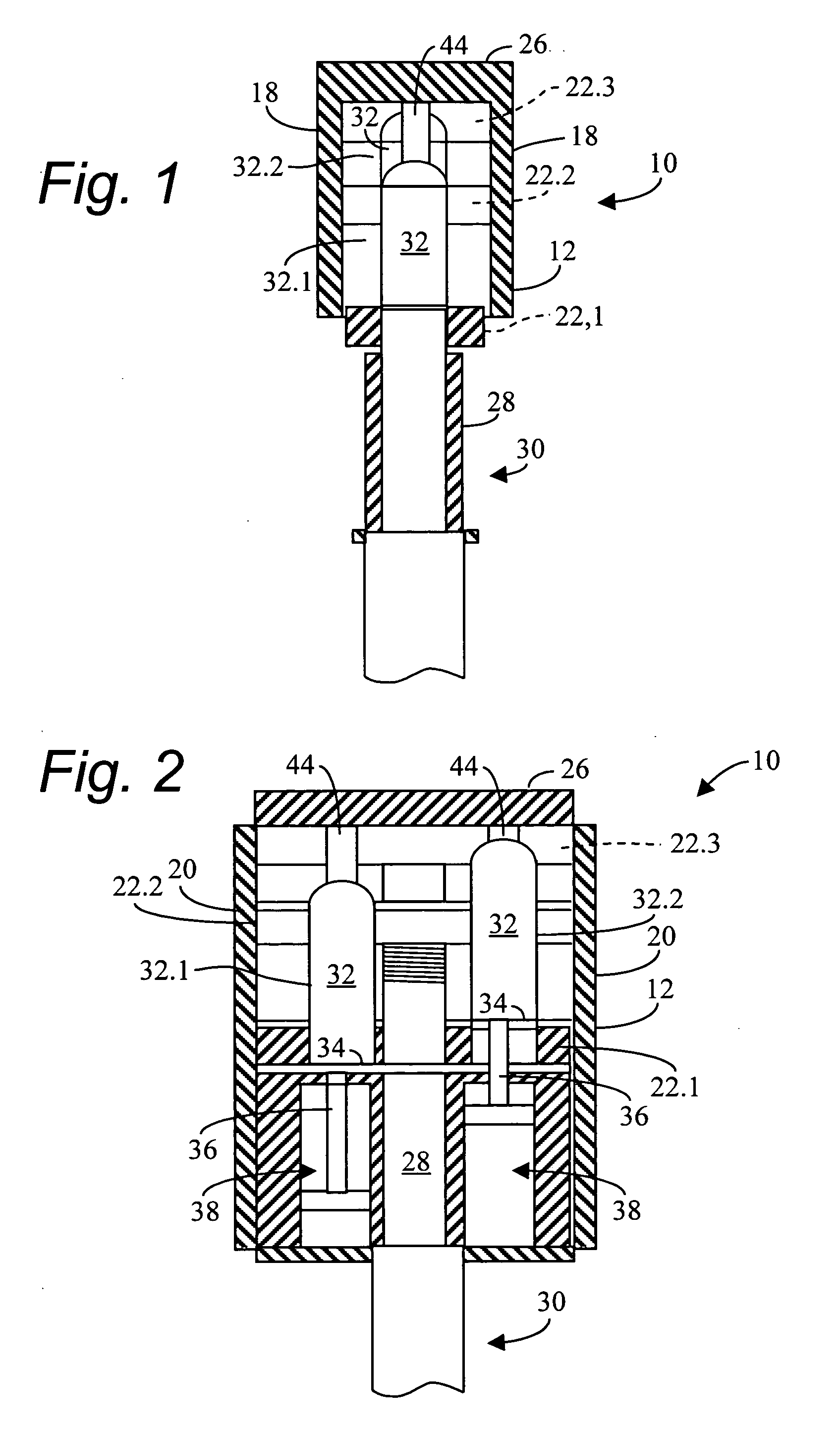
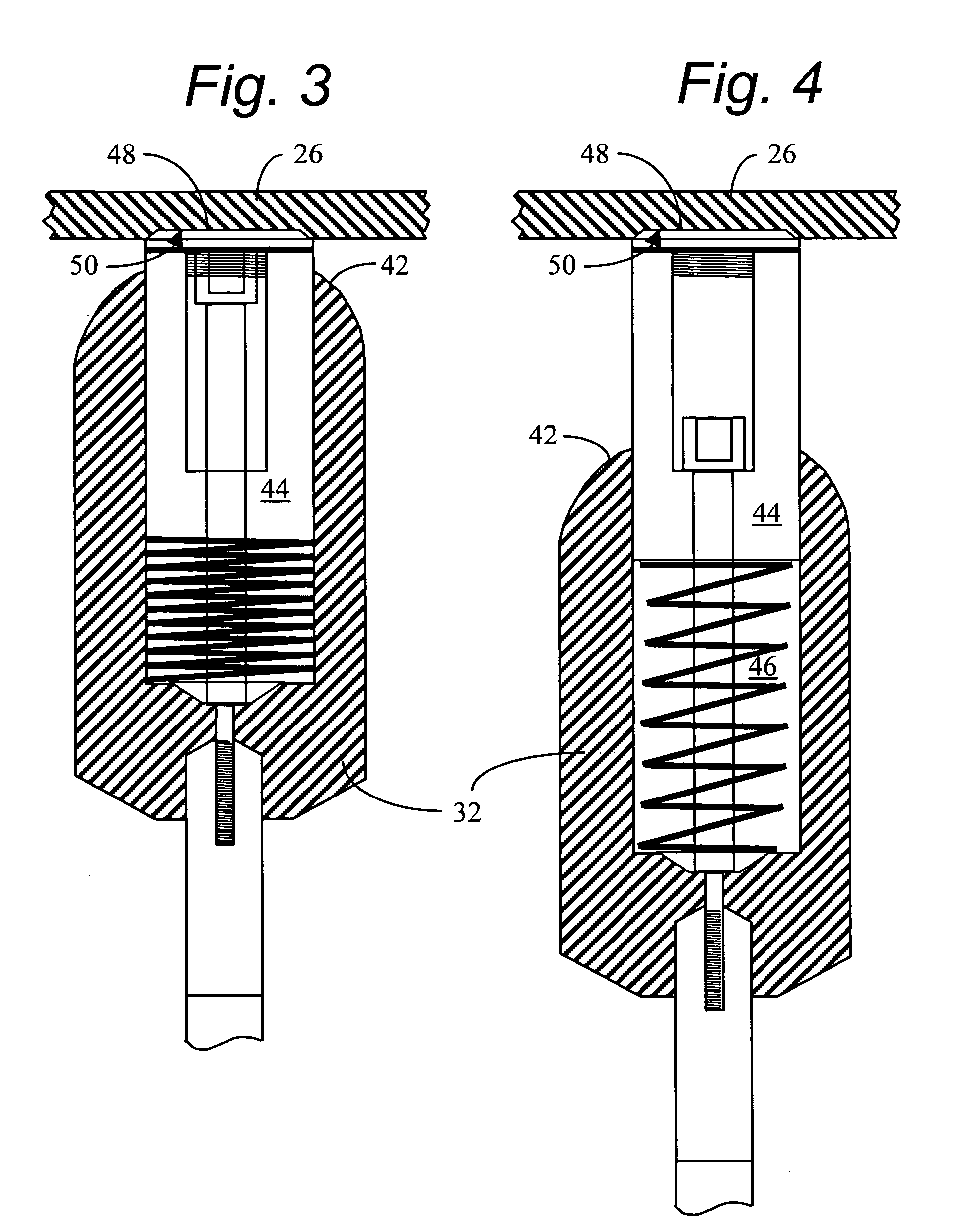
Method and apparatus for manufacturing compressed earthen blocks
InactiveUS20050029690A1High dimensional accuracyImprove homogeneityConstructionsConfectioneryHydraulic cylinderConstant stress
A machine and method for producing dimensionally consistent compressed earthen blocks under consistent and uniform pressures is disclosed. The approximately rectangular shaped block is formed in a rectangular parallelepiped shaped chamber having given dimensions. A plate forms one wall of this chamber and has the mobility required to compress the earth within the chamber. At the termination of compression, this plate is located at a predetermined location. A dog is forced into the compressed earthen block after the compression plate has ceased which effectively reduces the internal volume of the chamber. The dog is forced in under a known and consistent pressure. When a block is formed of less material, the terminal point for the dog will be further into the block than when the same-size block is made of more material. Additional aspects include a calibration unit for determining a volume of raw material to load into the compression chamber; and a hydraulic cylinder able to actuate two coaxial rams independently.
Owner:DYNABLOC TECH
Extrusion manufacturing process for hub outer ring
An extrusion manufacturing process for a hub outer ring comprises the following steps of preheating of a die, die closing and pressurizing, pressure maintaining, secondary pressurizing and water cooling. The extrusion die is preheated to prevent molten aluminum from forming a hard shell on the surface of the extrusion die; in extrusion casting, continuous pressure can eliminate the defects of castings. In the pressure casting process, medium-frequency heating is carried out on the extrusion die continuously, solidification and crusting in the extrusion process can be prevented, and an extrusion head can be made to compress a crystallizing layer continuously, so that liquid-phase metal in a shell layer is pressurized to further eliminate casting defects. Secondary pressurizing can mend the surfaces of the solidified castings and overcome the defects of the surfaces of the solidified castings, and the quality of the castings can be greatly improved. Before final demolding, the die is rapidly cooled, the surfaces of the castings can be rapidly cooled and actively contract inwards to be extruded, and separation stress is generated between the surfaces of the castings and the surface of the die, so that the surface quality of the castings cannot be affected in the demolding process.
Owner:重庆集飞智能装备技术有限公司
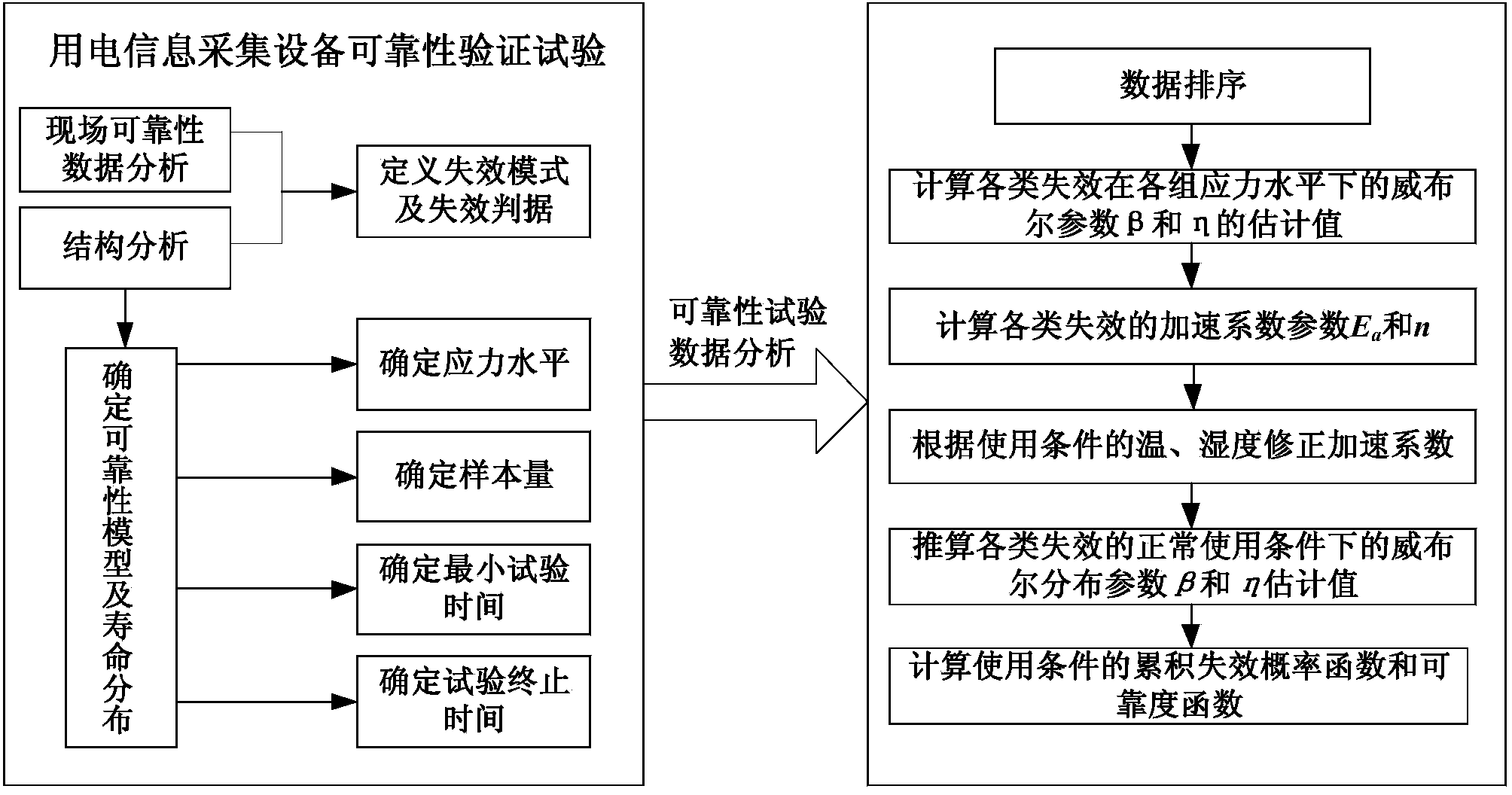
Testing method for verifying reliability of electricity utilization information collecting device
ActiveCN104076224AVerify reliabilityObtain full cycle life curveElectrical testingElectricityConstant stress
Owner:CHINA ELECTRIC POWER RES INST +2
Method for representing single-crystal Ni-base alloy creep resistance
InactiveCN105784508ASimple structureMethod is feasibleInvestigating material ductilityConstant stressSingle crystal
A method for representing single-crystal Ni-base alloy creep resistance comprises the steps of building a creep curve model suitable for single-crystal Ni-base alloy creep characteristics; determining model parameters to obtain a creep curve equation; performing creep data regression to obtain a fitting creep curve for describing a creep process; obtaining a creep rate equation, obtaining the minimum calculation creep rate and calculating a creep rate curve; combining specific requirements for representing the single-crystal Ni-base alloy creep resistance.Various single-crystal Ni-base alloy creep curves under constant temperature and constant stress except the extremely-high temperature or extremely-high stress and creep rate distribution can be accurately expressed; the corresponding relations of various creep curve equation items and creep parameters and alloy creep curves is revealed; the problem that some alloy cannot represent creep characteristics easily through the creep activated energy and stress indexes is solved; and the method is of great importance in further knowing the creep characteristics and rules of single-crystal Ni-base alloy.
Owner:SHENYANG POLYTECHNIC UNIV
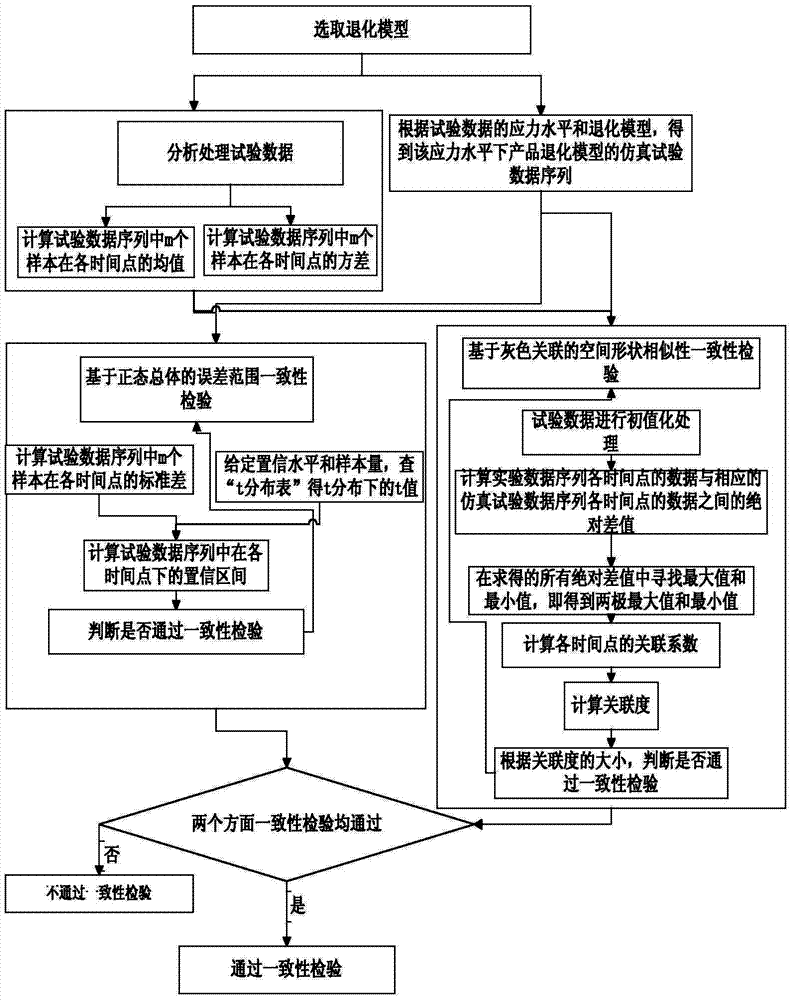
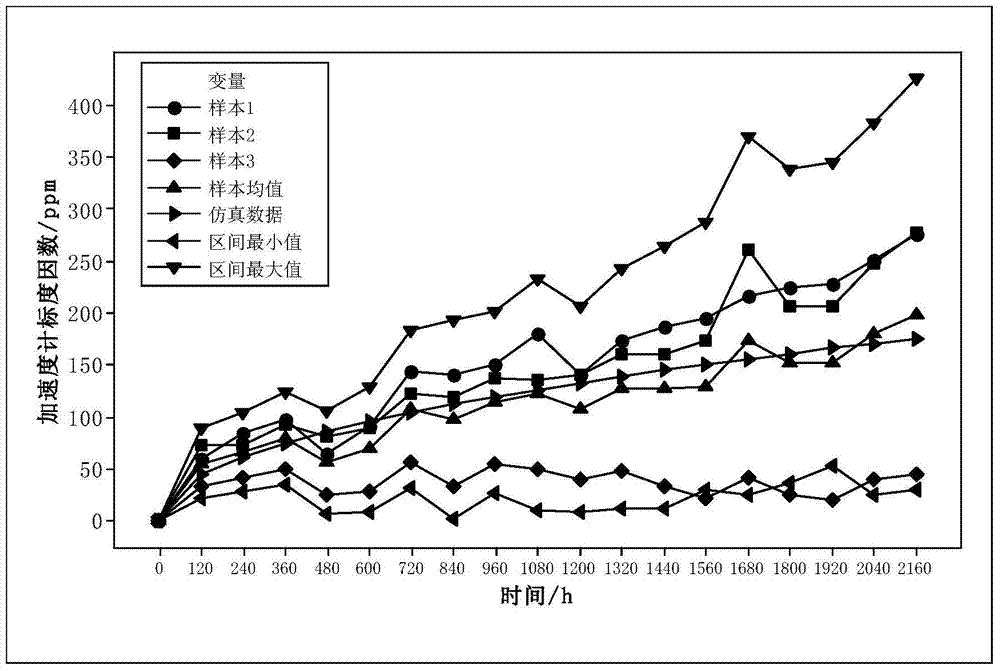
Degradation model consistency testing method catering to space shapes and error ranges
InactiveCN103678938AMake up for limitationsEasy to implementSpecial data processing applicationsData seriesAlgorithm
The invention discloses a degradation model consistency testing method catering to space shapes and error ranges. The method includes the following steps that firstly, a product degradation model is selected; secondly, test data are analyzed and processed, wherein the test data are m data series {y[i](t), t=1,2,...,N, i=1,2,...,m} of performance parameters and time of m products under a constant-stress level; thirdly, a simulation test data series of the product degradation model is acquired under the stress level in the form of {x(t), t=1,2,...,N} according to the stress level of the test data and the degradation model; fourthly, error range consistency testing is carried out based on normal populations; fifthly, space shape similarity consistence testing is carried out based on grey correlation; sixthly, whether a degradation model caters to error range consistency testing based on the normal populations and space shape similarity consistency testing based on grey correlation or not is judged, if yes, the degradation model passes consistency testing, and otherwise the degradation model does not pass consistency testing. The degradation model consistency testing method overcomes the defect that only model error ranges are considered in existing methods and is easy and convenient to implement and wide in application range.
Owner:BEIHANG UNIV
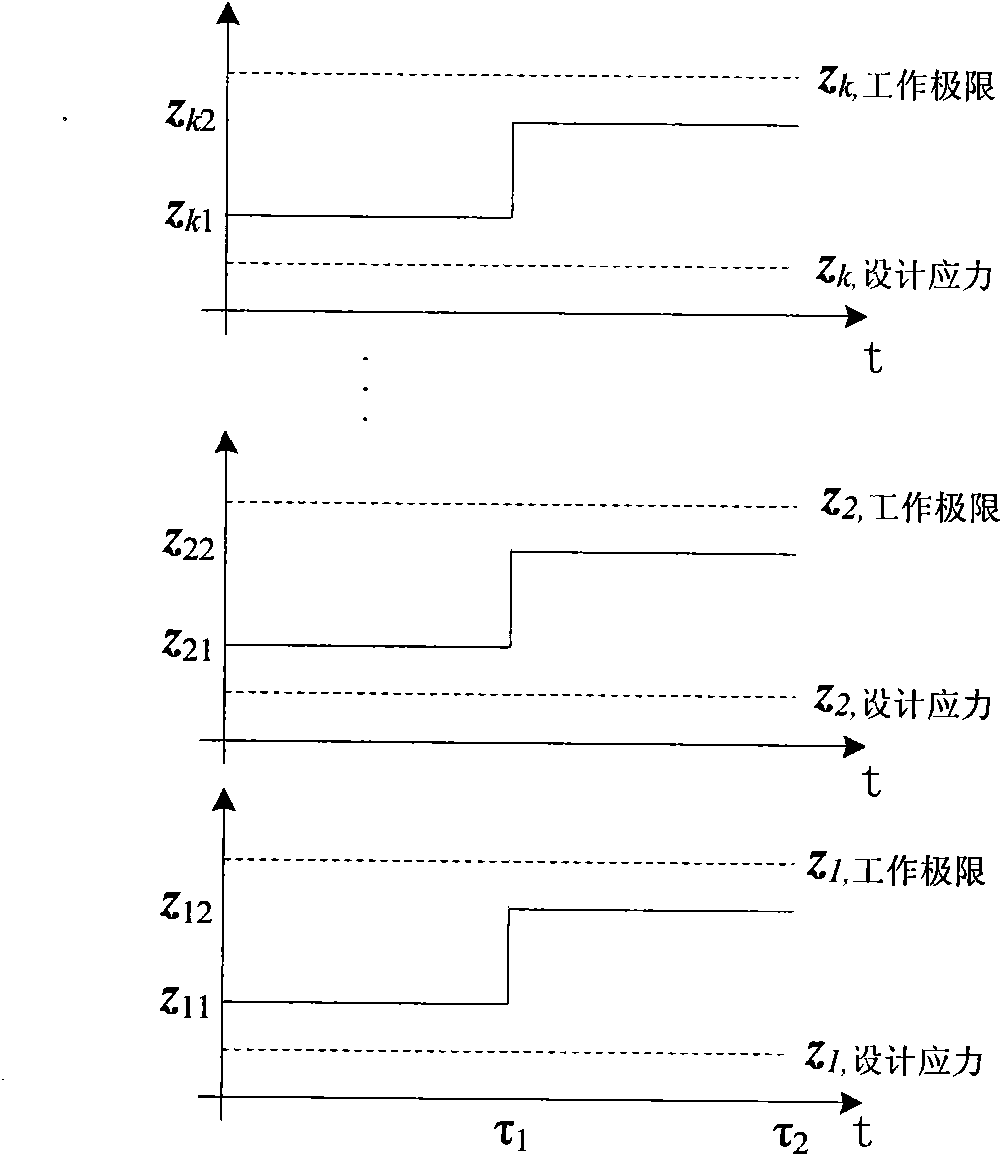
Ring shear apparatus considering constant stiffness, constant volume and constant stress,
The invention discloses a ring shear apparatus considering constant stiffness, constant volume and constant stress, wherein the ring shear apparatus includes a rack, a shearing system, a torsion device, a hydraulic loading system and the like. The shearing system comprises an upper shearing box, a lower shearing box and a stiffness adjusting device. The stiffness adjusting device is arranged abovethe upper shearing box, and the hydraulic loading system and the torsion device are respectively arranged above and below the shearing system; the hydraulic loading system is connected with the stiffness adjusting device to apply the stress to the shearing system; the torsion device is used for providing torque to the lower shearing box. Compared with a conventional ring shearing apparatus, the ring shear apparatus can adjusting the stiffness state of the device by adjusting and controlling the stiffness, and can realize shearing states of three different constraint conditions of constant stiffness, constant volume and constant stress by combining the loading condition of the hydraulic loading system. Shear test detection of different contact types between soil and interfaces can be achieved by an experimental device; a number of mechanical parameter indexes can be obtained, parameters can be provided for engineering design and installation, and engineering practice is guided.
Owner:ZHEJIANG UNIV
Reliability life evaluation method of long-life electronic device under multiple stresses based on depth belief network
InactiveCN108664690AImprove applicabilityImprove general performanceDesign optimisation/simulationNeural architecturesDeep belief networkStatistical analysis
The invention discloses a reliability life evaluation method of a long-life electronic device under multiple stresses based on a depth belief network, which estimates reliability characteristics by obtaining device failure data under different environmental stresses through an accelerated life test, and then establishes a reliability derivation model to complete the reliability life derivation under a constant stress level. The evaluation method comprises the following steps: firstly, determining the environmental stress which affects the working reliability of the electronic components; secondly, designing the accelerated life test; thirdly, performing statistical analysis in the test data to obtain the reliability life estimated values of components under different stress levels; and finally, determining the reliability derivation model by using the accelerated life test data. The invention solves the problems of sample shortage and limited test time, adopts a novel constant stress reliability characteristic derivation model, solves the problem that the traditional single stress estimation method is prone to model nesting risk, and realizes the establishment of the life derivation model under multiple stress levels.
Owner:BEIJING UNIV OF TECH
Dynamic fatigue evolution test method for rubber and fiber cord thread bonding
InactiveCN104502276AAccurately reflectEasy to operateUsing mechanical meansMaterial analysisElastomerFiber
The invention discloses a dynamic fatigue evolution test method for rubber and fiber cord thread bonding, by means of connecting a fixture with an MTS elastomer test system, the method realizes simulation of continuous evolution process of bonding failure of rubber and a fiber cord thread under various conditions of use in multiple modes such as constant stress, constant strain as well as alternating load and alternating frequency. The beneficial effects of the method are that: the method is simple to operate, the data is reliable, the reproducibility is high, the test process has time continuity, so the method can better simulate the evolution process of bonding fatigue rupture of multiple kinds of rubber and various fiber cord thread composite materials under the condition of real stress, and has important guiding significance for predicting the service life of rubber products,thereby having a broad application prospect.
Owner:QINGDAO UNIV OF SCI & TECH
Leak detection equipment
InactiveCN105203278AIndicates air leaksPrevent missed detectionMeasurement of fluid loss/gain rateConstant stressDirect observation
The invention discloses leak detection equipment for a flexible packaging product. The leak detection equipment comprises a rack and pressurization equipment. The pressurization equipment is an air cylinder piston assembly capable of applying pressure on the flexible packaging product continuously, and the fixed end of the air cylinder piston assembly is fixedly connected with the rack. A detection platform where the flexible packaging product to be detected is placed is further fixedly arranged on the rack. The air cylinder piston assembly is connected with a pressure gage used for detecting pressure of an air cylinder. The air cylinder piston assembly adopted for pressurization can output pressure continuously with the pressure maintenance characteristic, and whether the packaging product leaks air can be detected more thoroughly through pressure detection, with a certain time length, on the packaging bag; besides, pressure changes of the air cylinder are detected through the connected pressure gage, pressure changes in the packaging product pressurized by the air cylinder are displayed accordingly, and therefore the sealing air leak condition of the flexible packaging product can be correctly reflected; manual direct leak observation is replaced with reading of the pressure gage, and therefore the leak detection equipment is more reliable and saves labor.
Owner:长沙汇一制药机械有限公司
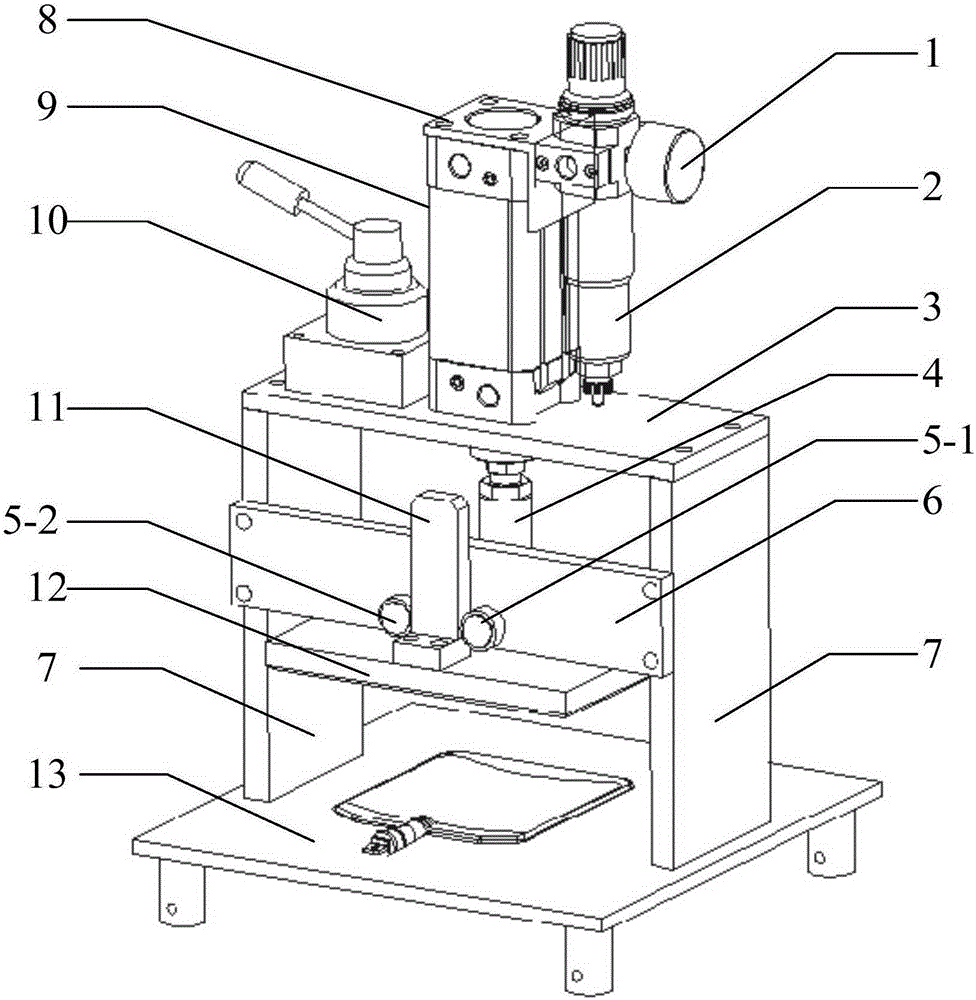
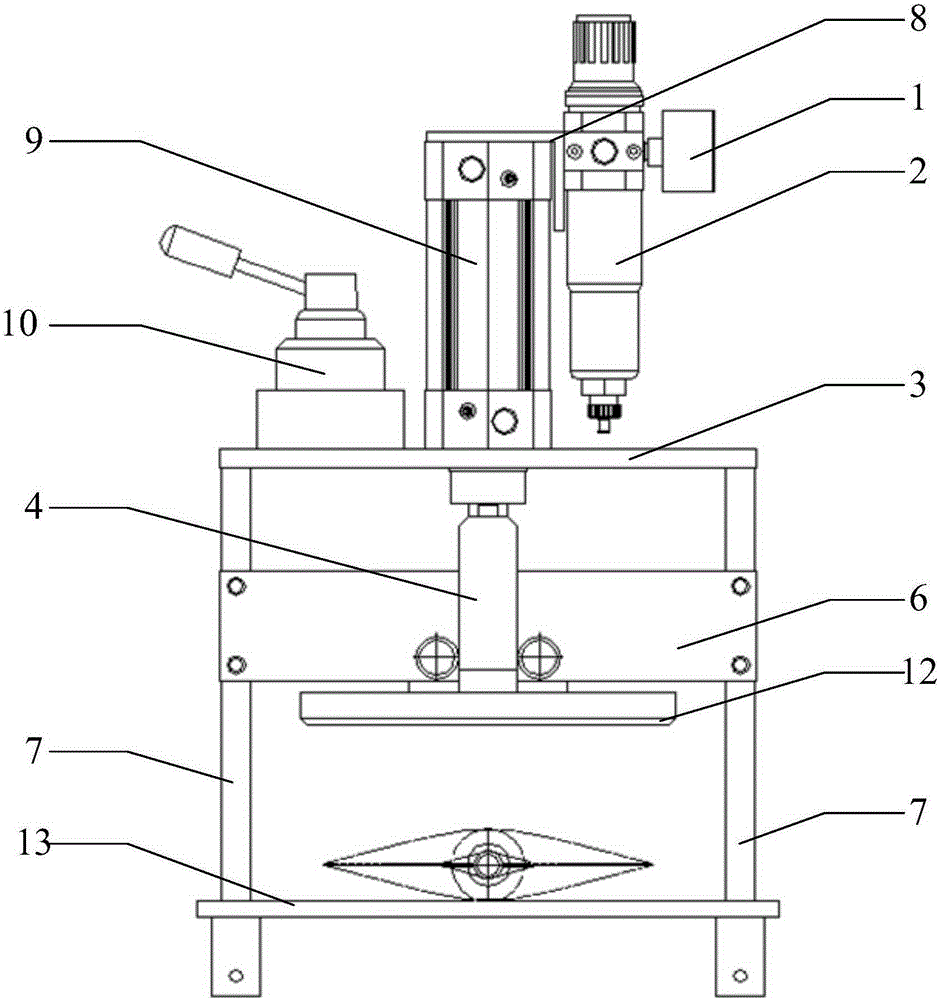
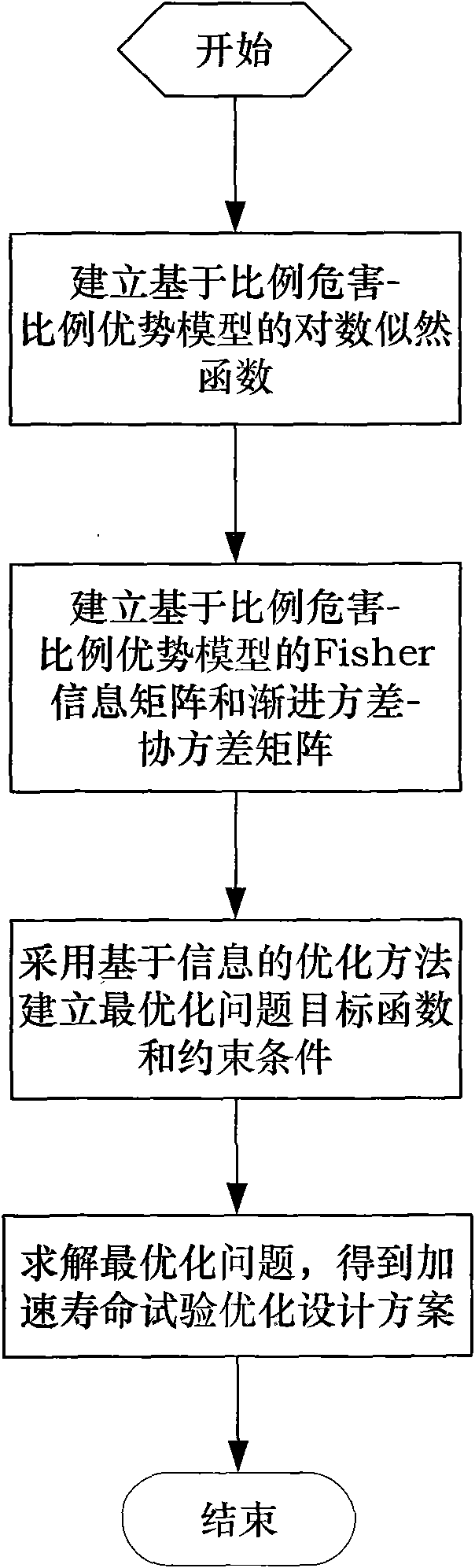
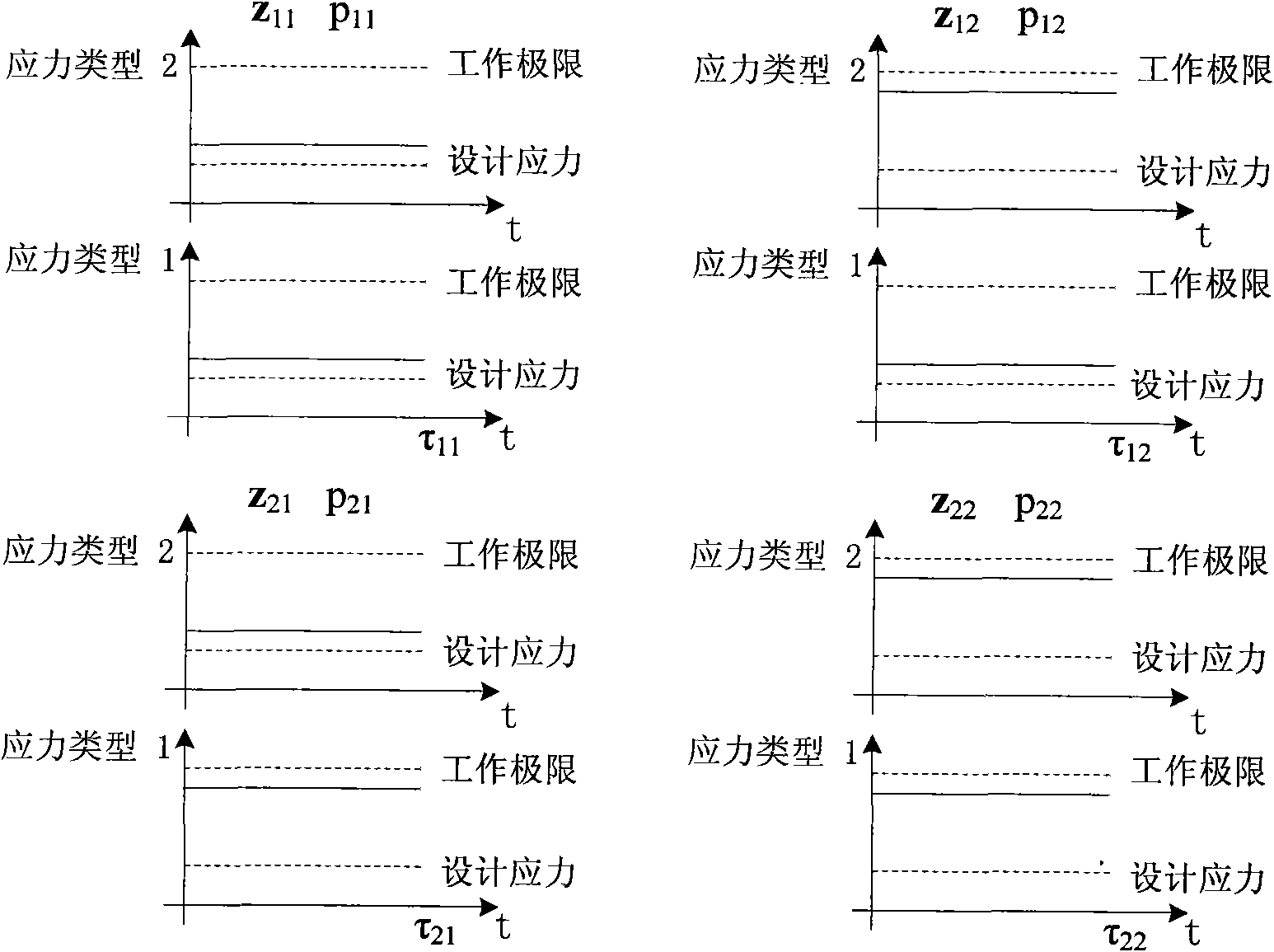
Accelerated life test optimization design method based on proportional hazards-proportional odds model
InactiveCN101620034ANo distribution characteristicsAvoid the problem of inconsistent optimization resultsStructural/machines measurementProportional hazards modelConstant stress
The invention discloses an accelerated life test optimization design method based on a proportional hazards-proportional odds model. The method comprises the following steps: establishing a logarithmic likelihood function based on the proportional hazards-proportional odds model; establishing a Fisher information matrix and an asymptotic variance-covariance matrix based on the proportional hazards-proportional odds model; establishing an objective function and constraint conditions of an optimization problem by adopting an information-based optimization method; and obtaining an accelerated life test optimization design scheme by solving the optimization problem. The invention provides an optimization design method for accelerated life test of constant stress and step stress, the model adopted during the accelerated life test optimization design is a proportional hazards-proportional odds model, the model has wider application range and higher evaluation precision than the proportional hazards model and the proportional odds model; the model is a nonparametric model and has the distribution-free characteristic; and the method avoids the problem of inconsistent optimization results caused by integral interval change in the traditional optimization method.
Owner:BEIHANG UNIV
Load applying method of refractory material tester
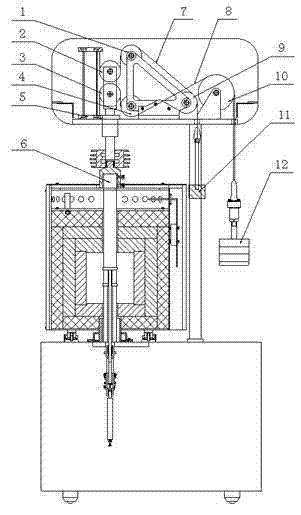
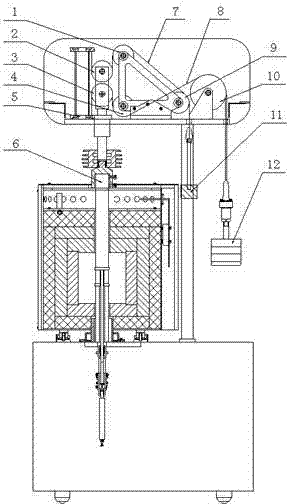
ActiveCN102346112AReduce tensionImprove accuracyInvestigating material ductilityRefractoryConstant stress
The invention discloses a load applying method of a refractory material tester. A movable pulley mechanism is used for applying constant stress; two groups of stress transferring mechanisms consisting of movable pulleys, fixed pulleys and flexible steel wire ropes respectively share the action of load application and balance adjustment, wherein the two movable pulleys form a movable pulley block, and a central shaft of crossed and branched chains is connected with a pressurizing rod; and one end of each of the two steel wire ropes is respectively connected with an applied load and a balance hammer. The method has the advantages that the load is applied by a movable pulley structure, so that the load and the mass for adjusting balance are greatly reduced, and the adjustment and the operation are more convenient; the test error is reduced; the service life of the steel wire ropes is correspondingly prolonged; the labor intensity of an operator is greatly reduced, and the safety of staffs and equipment is greatly improved; and the load application and the balance adjustment are respectively shared by the two flexible steel wire ropes, respective adjustment is realized and the operation is flexible.
Owner:SINOSTEEL LUOYANG INST OF REFRACTORIES RES
Method for creating a Low Fluid Pressure Differential Electrical Generating System
InactiveUS20090178462A1Useful for promotionReduce liability riskGas-turbine engine testingFluid pressure measurementGenerators (Apparatus)Engineering
This invention involves a method of creating a hydroelectric turbine system that is capable of producing electricity while sustaining a majority of the fluid flow or hydraulic pressure within the pipe line or pipe network driving the system. The system's objective is to shave as little pressure as possible from an enclosed pipe system that requires pressure to operate and function properly. More particularly, this invention relates to a hydroelectric turbine generator system wherein the system is specially designed and configured with an unique impeller and fluted turbine housing that enable a generator device to produce a particular amount of electric current and voltage, while yet minimizing flow restriction and pressure loss to the fluid pressure driving the system. Application of this technology includes all fluid flow or fluid transfer systems that require sustained pressure to move fluids like water or other materials from one location or point to another, through pipes, pipe systems or networks, regardless of distance or pipe size diameters. This invention, for example will be ideally suited for powering electronic control or monitoring systems in landscapes, parks, roadway easements, golf courses or public water transfer systems or other remote sites that may require power. Utilization of this invention provides an ecological and reliable energy production source, usually on a small scale, in remote locations where incorporating other forms of power supply may be less practical, impractical or impossible.
Owner:TECHSTREAM CONTROL SYST
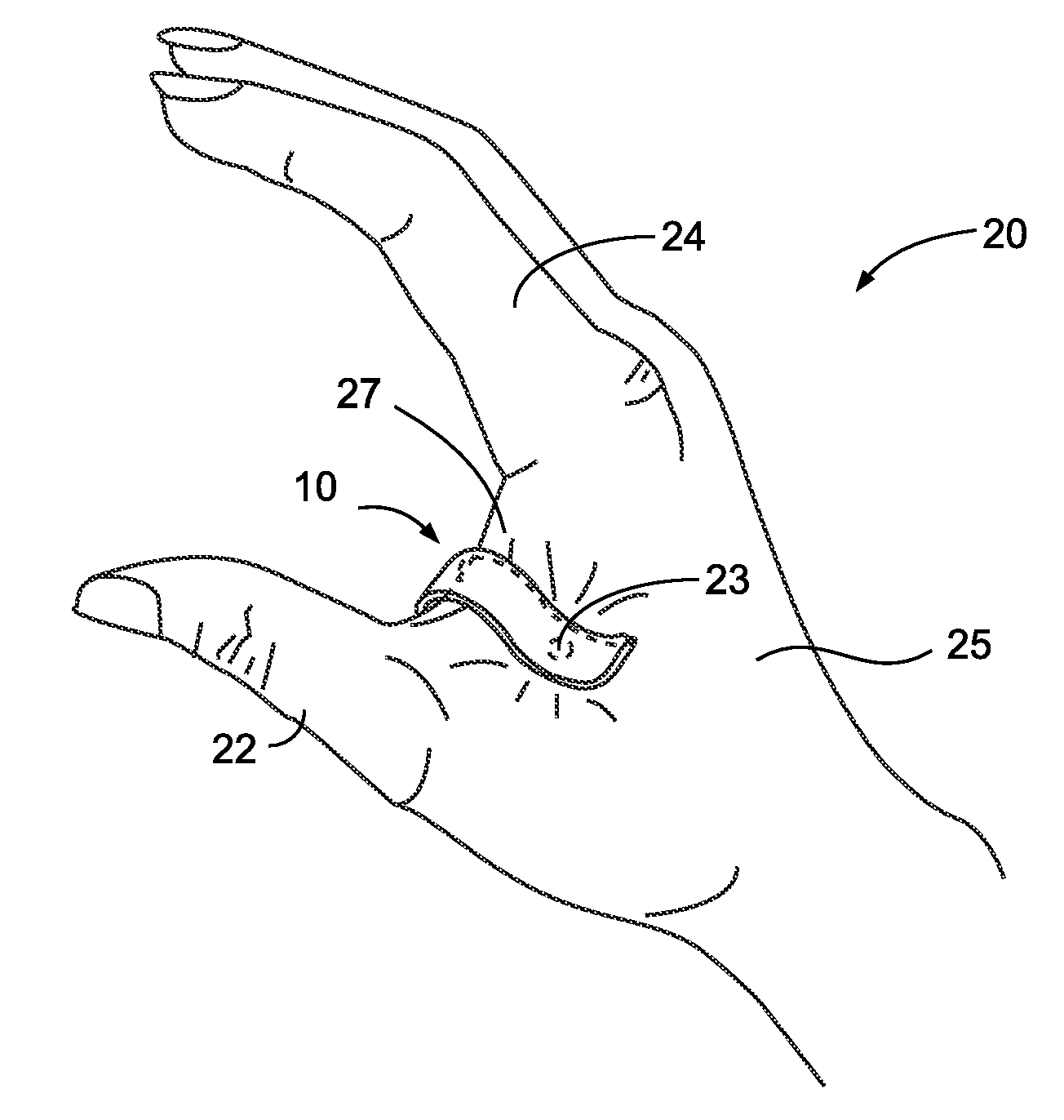
Acupressure Treatment Device
InactiveUS20070093867A1Easy to installEasy to snagDevices for pressing relfex pointsSurgeryAcupressureEdge surface
An acupressure clip for relieving a user's headache pain is disclosed. A generally U-shaped clip member includes an active arm and a support arm. Each arm meets at a spring portion, and each arm and spring portion include a continuous outside surface, a continuous inside surface, and at least one continuous edge surface. A pressure nub projects from the inside surface of the active arm and extends towards the supporting arm at a point closest to the supporting arm. In use, the clip is positioned on the user's hand between the thumb and the forefinger such that the pressure nub contacts and applies continuous pressure to the LI-4 acupressure point of the person's hand to relieve the person's headache pain. The clip is retained on the hand through friction and the spring force caused by separating the two arms, thus allowing uninhibited use of the hands.
Owner:SAVOIA RON
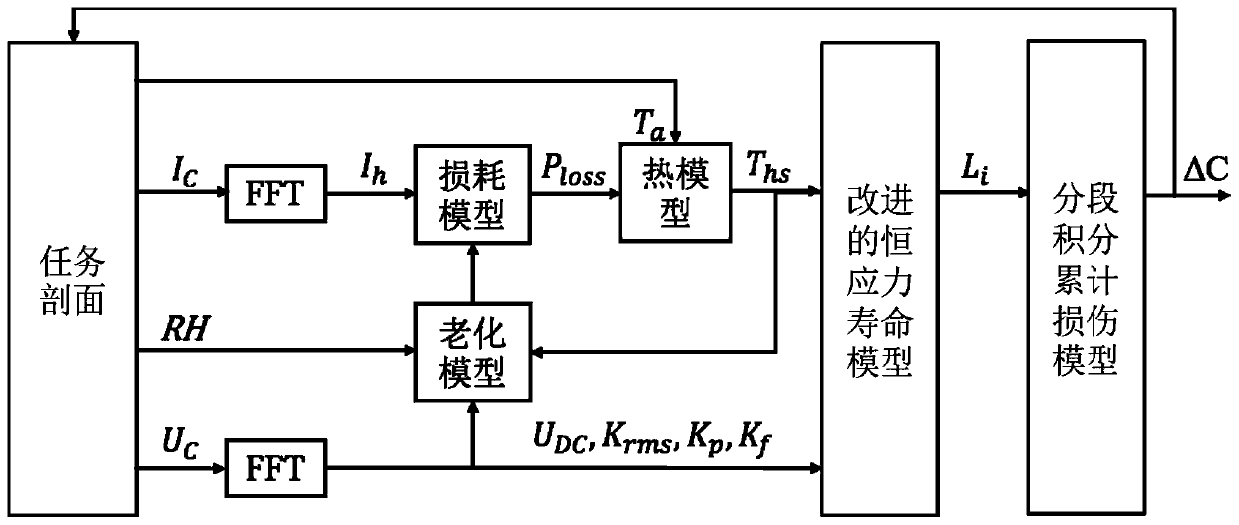
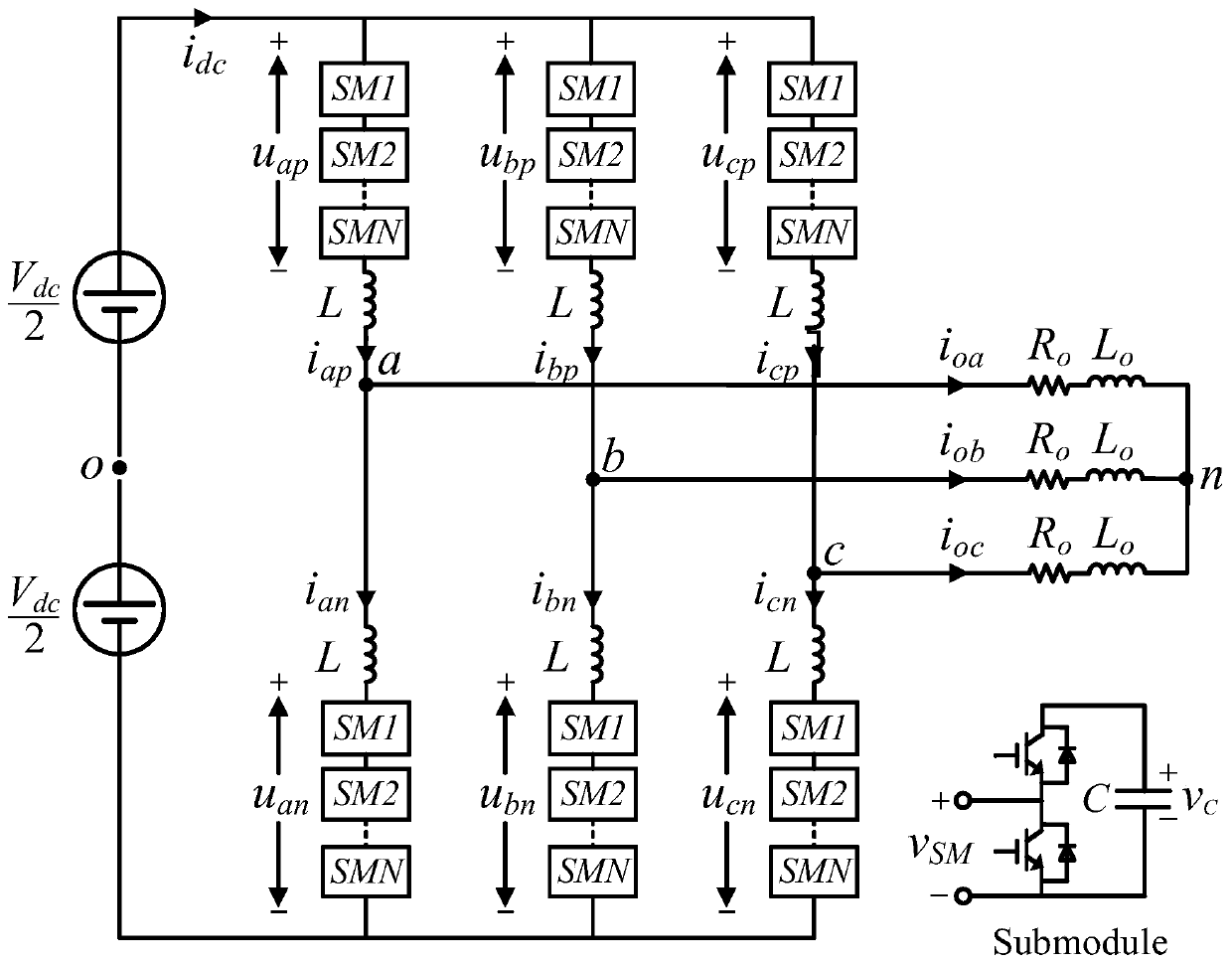
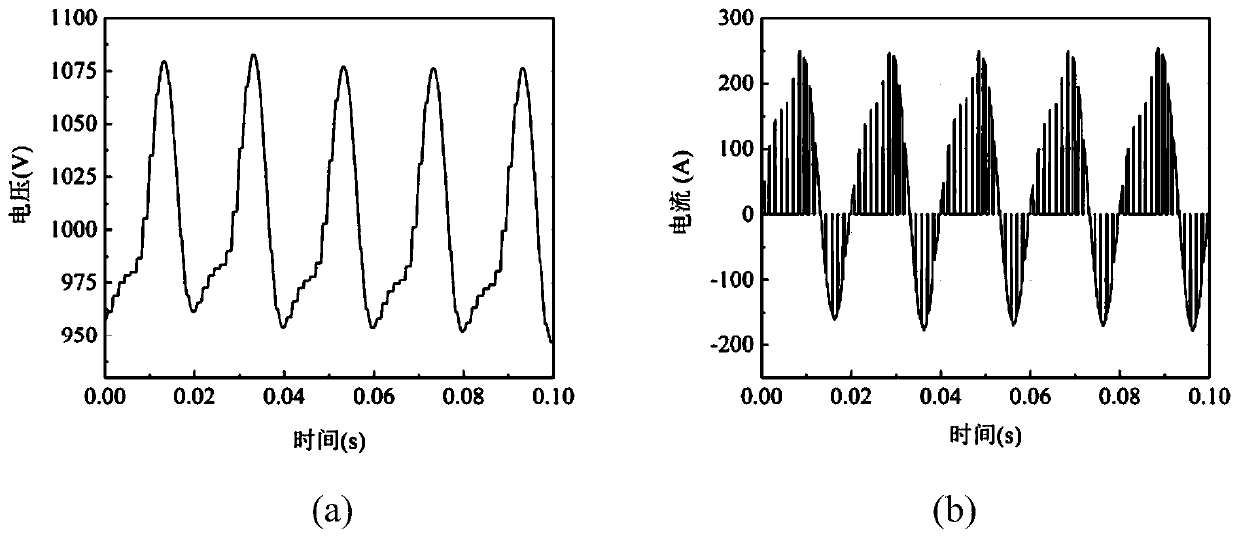
Method for predicting service life of metallized film capacitor based on mission profile and aging analysis
ActiveCN111104756AImprove reliabilityThe life model is accurateDesign optimisation/simulationCAD numerical modellingCapacitanceElectric power
The invention discloses a method for predicting the service life of a metallized film capacitor based on a mission profile and aging analysis. The method comprises the following steps: constructing aconstant-stress life model, and obtaining the expected life of the metallized film capacitor under a constant-stress experiment condition; constructing an aging model, considering that internal parameters and stress change under the long-time scale operation of the metallized film capacitor, and obtaining the degradation curve of a capacitor capacitance value and equivalent series resistance undera constant stress experiment working condition; and constructing a segmented integral accumulation damage model, analyzing nonlinear aging caused by changes of internal and external stresses under actual working conditions, and obtaining the expected service life of the metallized film capacitor under the actual working conditions. According to the method disclosed by the invention, the life analysis is performed on the metallized film capacitor and the expected life is obtained by considering the actual operation condition and the capacitor degradation process, and a reference is provided for improving the reliability of the power electronic converter.
Owner:XI AN JIAOTONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com