Transfection reagents
a technology of transfection reagents and reagents, applied in biochemistry apparatus and processes, instruments, material analysis, etc., can solve problems such as difficult conventional treatment, and achieve the effect of increasing cross-sectional area and reducing the hydrophobicity of reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
EDLPC / EDOPC Transfection Reagents
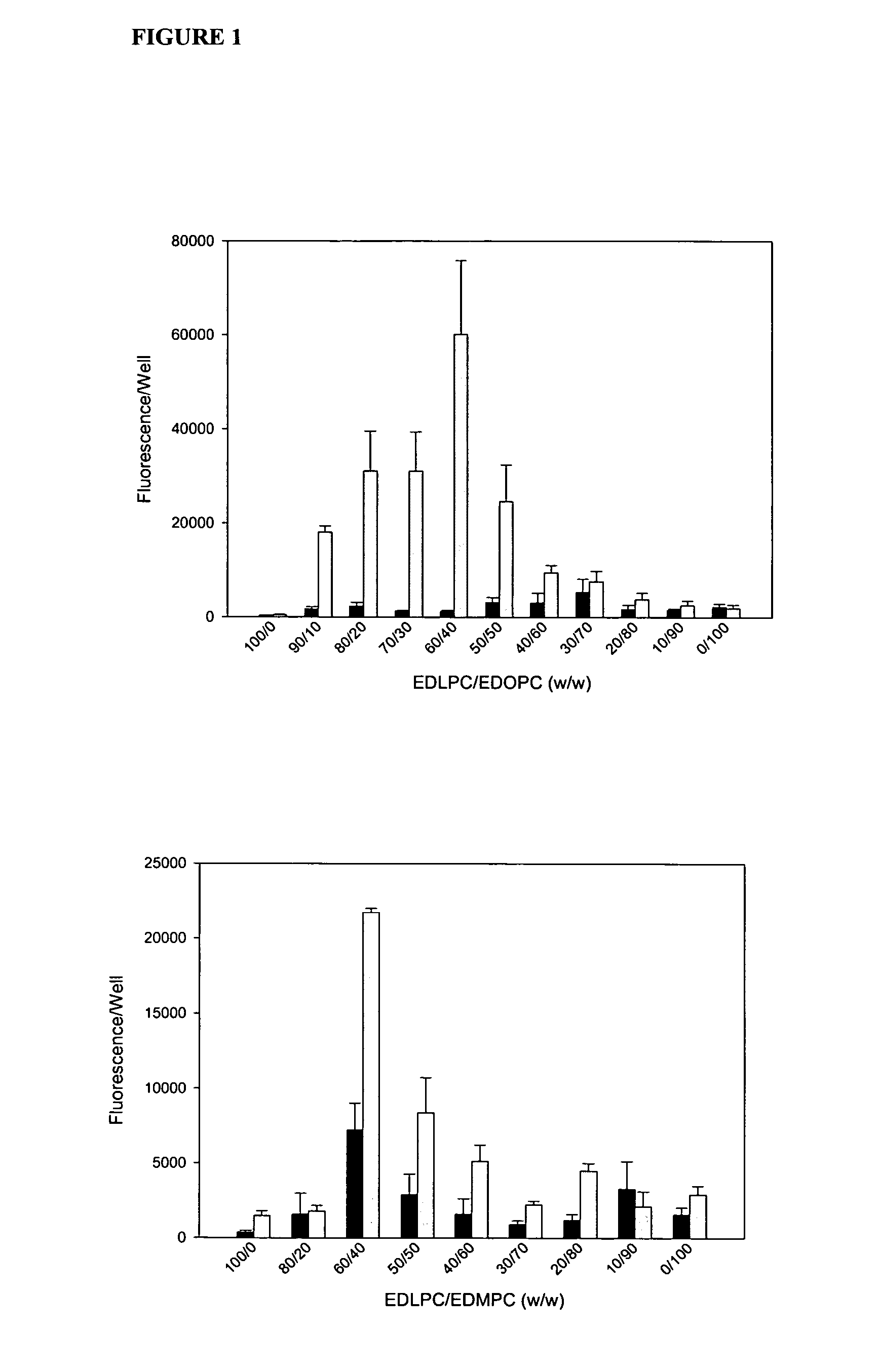
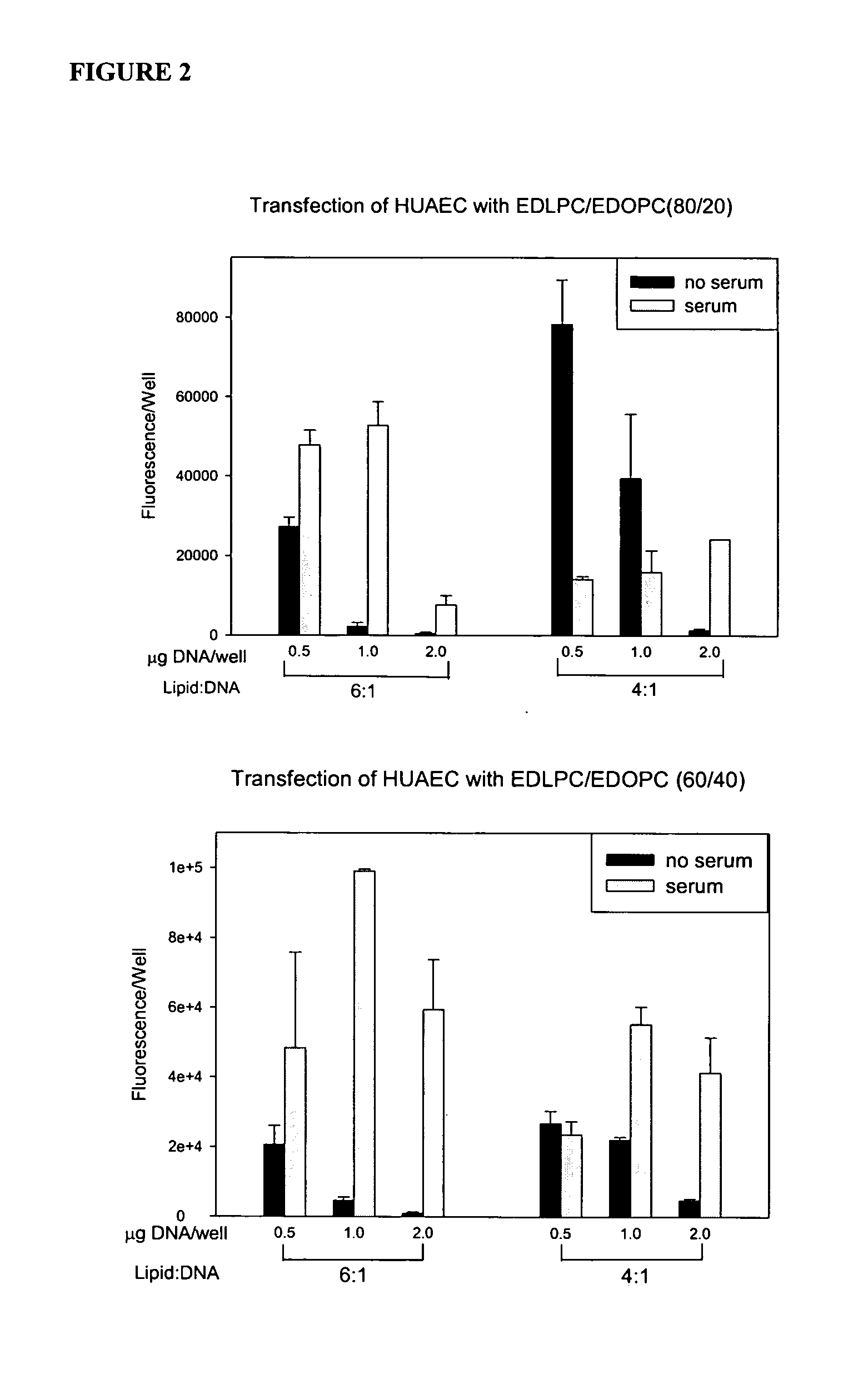
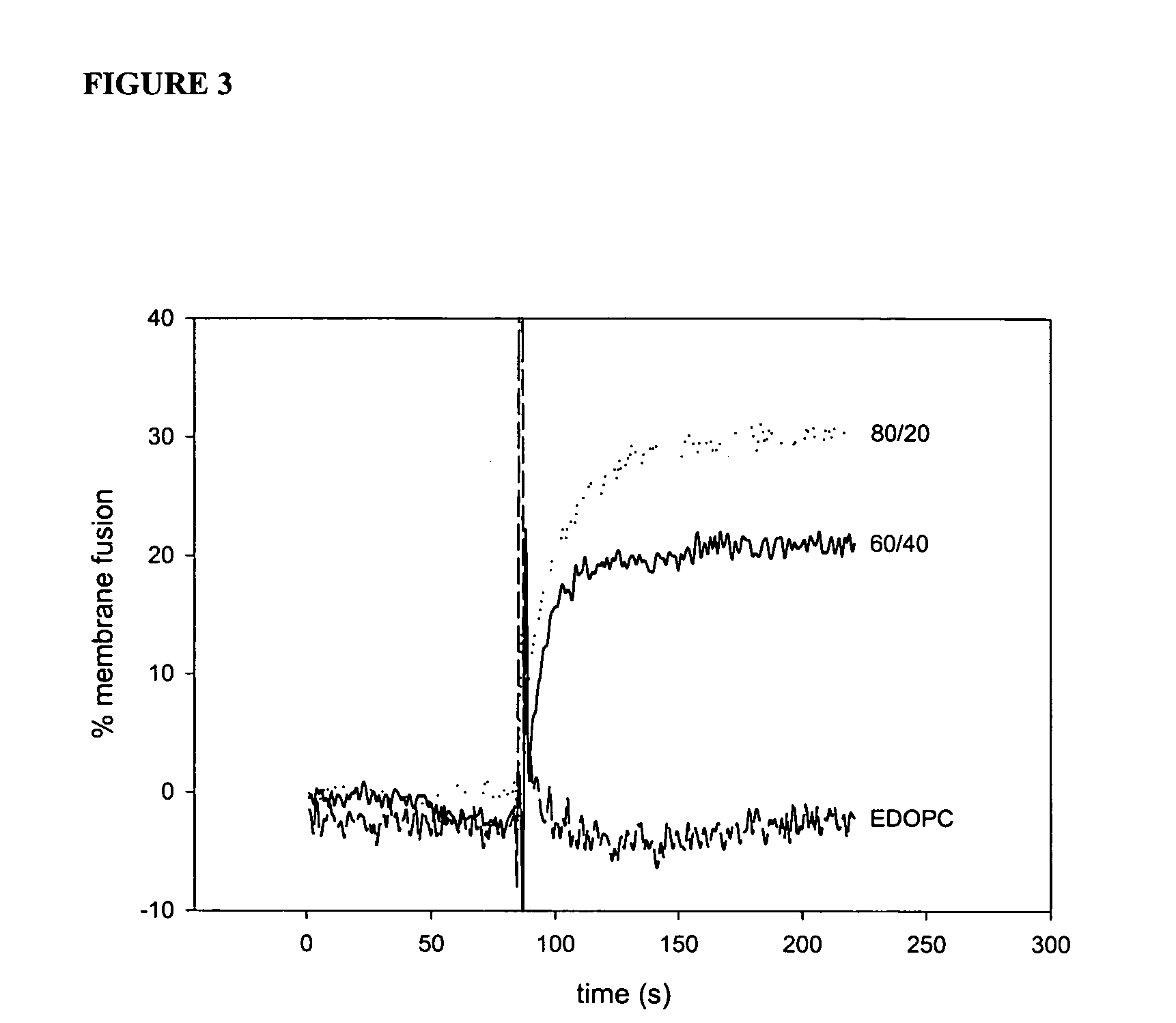
[0072] Experiments conducted during the development of the present invention found that attention to the hydrophobic portions of medium and long-chain cationic lipids synergistically enhance transfection. It was found that a combination of two cationic lipid derivatives with the same head group but tails of different chain lengths behave considerably differently as transfection agents than the separate molecules. For example, the combination of the dilauroyl (12 carbon chain) and the dioleoyl (18 carbon chain) homologues of O-ethylphosphatidylcholine transfected DNA into primary human umbilical artery endothelial cells (HUAECS) more than 30-fold more efficiently than either compound separately. The present invention is not limited to a particular mechanism. Indeed, an understanding of the mechanism is not necessary to practice the present invention. Nonetheless, these results suggest that the hydrophobic portions of medium and long-chain cationic li...
example 2
Transfection of Human Dermal Fibroblasts with EDOPC / EPOPC and EDOPC / EDiphytanoyl PC Transfection Reagents
[0087]FIG. 8 shows that combining EDOPC with EPOPC (one oleoyl chain, which is 18C's with one double bond, and one palmitoyl chain, which is 16C's without any double bond) shows little mixing effect in transfection of human dermal fibroblast cells in the absence of serum.
[0088]FIG. 9 shows that combining EDOPC with EDiphytanoyl PC (two phytanoyl chains, 16 carbon chains with 4 methyl branches) shows marked mixing effect in the transfection of human dermal fibroblast cells in the absence of serum.
example 3
Transfection of HUAECs with EDOPC / EPOPC, EDOPC / EDiphytanoylPC, EDOPC / SDOPC and EDOPC / EC18C10PC
[0089]FIG. 10 shows the results of transfecting HUAECs with EDOPC / EPOPC, EDOPC / EDiphytanoylPC, EDOPC / SDOPC (DOPC with an 18 carbon chain instead of an ethyl group on the phosphate oxygen), and EDOPC / EC18C10PC mixtures.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com