Novel method for the protection and purification of adenoviral vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
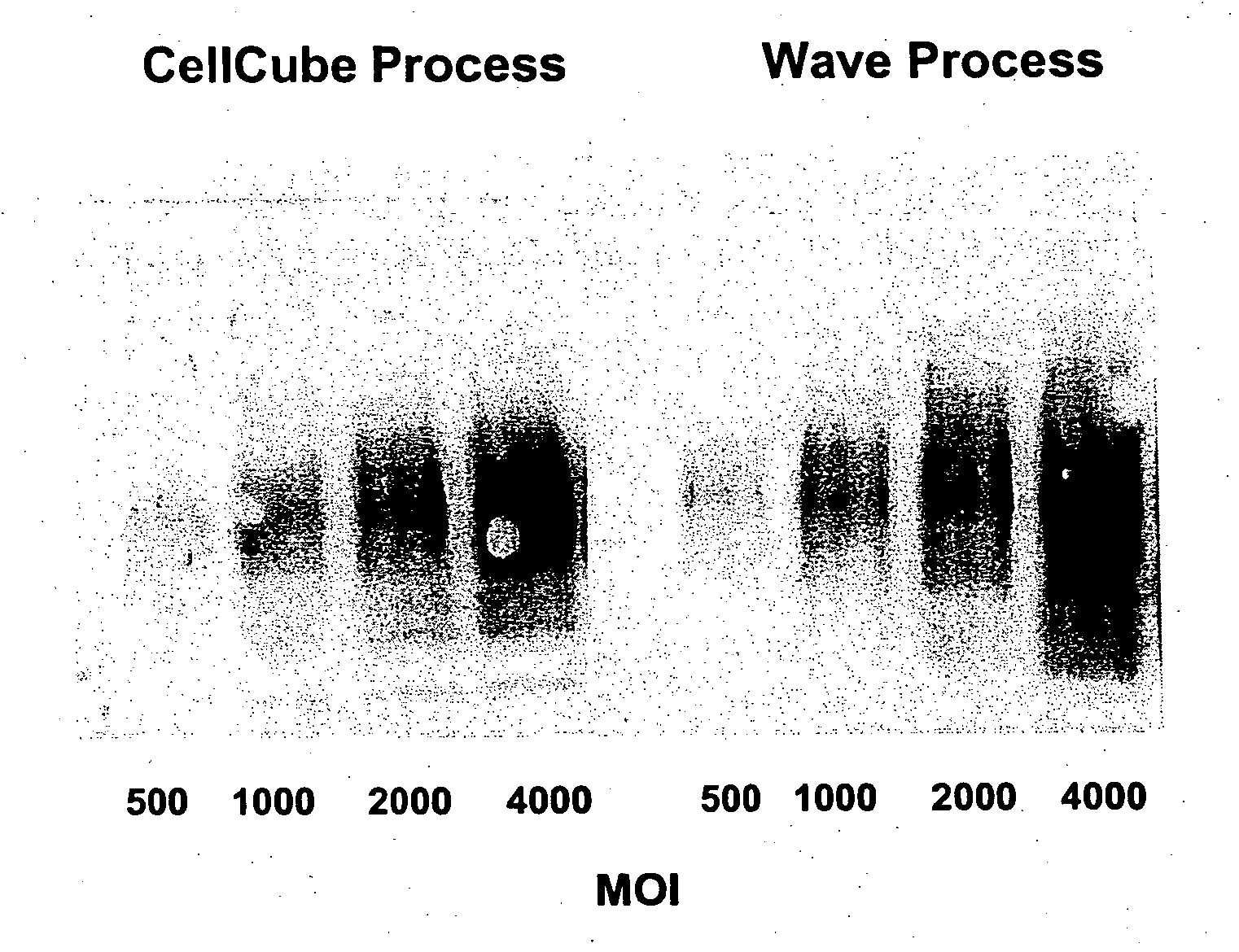
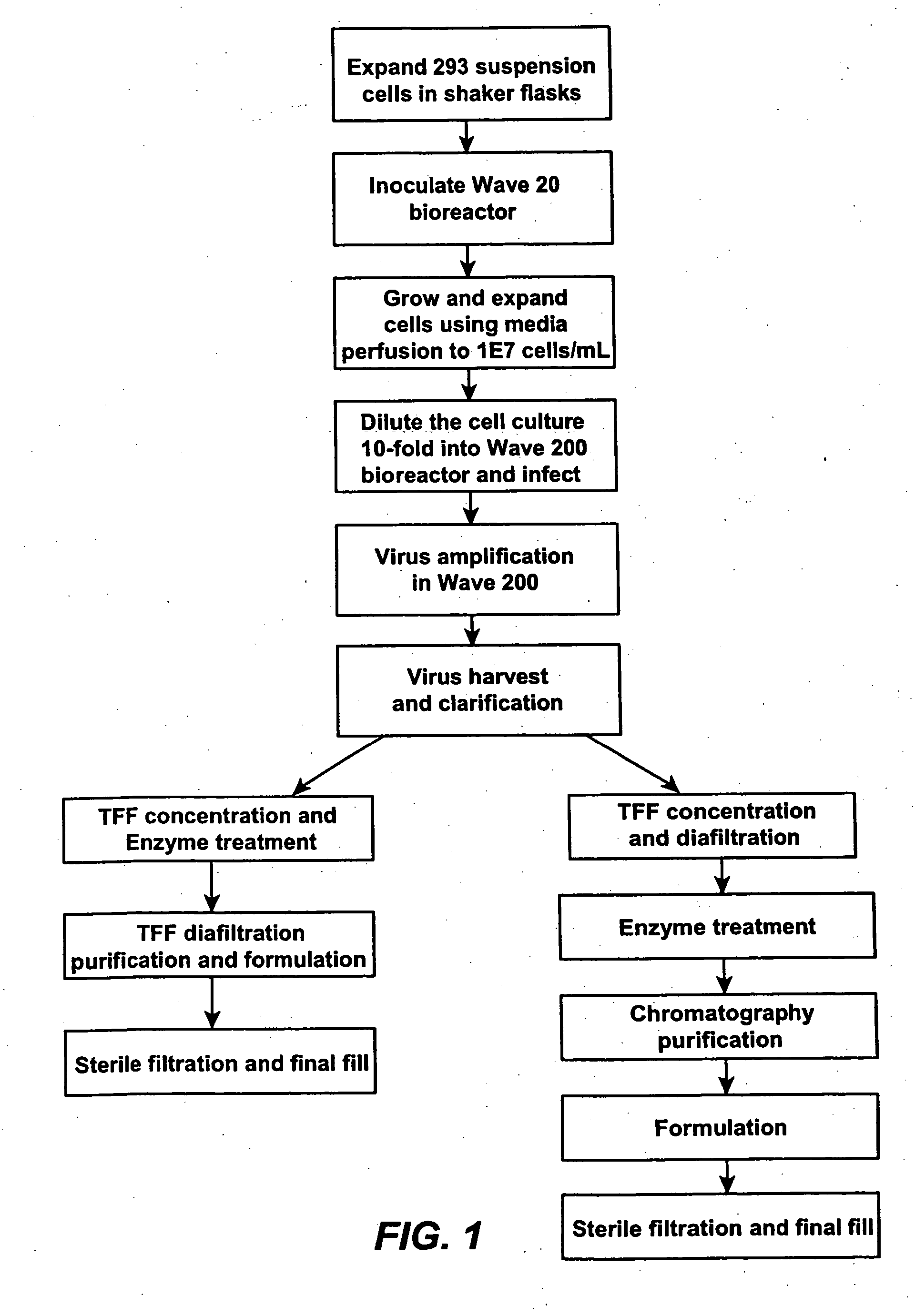
[0308] According to this example, cells were cultured and adenoviral vectors produced with medium perfusion using a 10 L (5 L working volume) Wave Bioreactor® (20 / 50EH (Wave Biotech, LLC) equipped with a YSI-2700 SELECT™ biochemistry analyzer according to the production and purification process depicted in FIG. 1. FIG. 3 depicts a perfusion Wave bioreactor (10) comprising an inflated plastic bag (12) containing cell culture media (14) and an internal flat perfusion filter (16) to provide separation between the cells and spent medium. Media is fed to the bioreactor from a feed bag (18) by feed pump (22) Spent culture medium is withdrawn through the floating filter (16) to a harvest bag (20) by harvest pump (24). Controller (26) controls the functions of the pumps and bioreactor (10). No medium recirculation is required, and consequently this mode of medium perfusion is very gentle to the cells in culture. The wave action minimizes filter clogging during perfusion. The culture volume ...
example 2
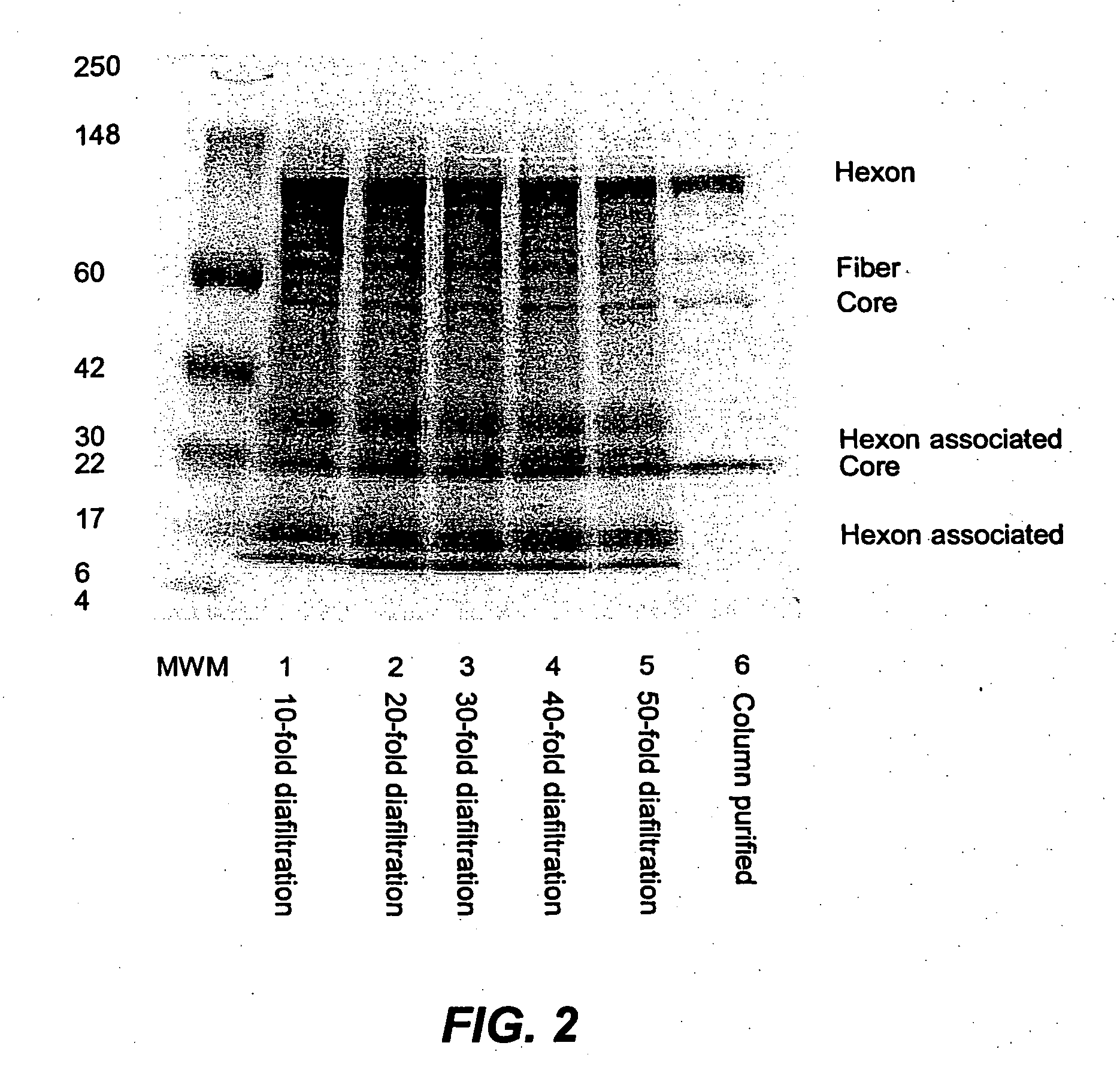
[0312] According to this example, the product of Example 1 was subjected to diafiltration using a tangential flow filtration (TFF) membrane using a Pellicon 2 mini system fitted with a 500 KD Biomax membrane cassette The clarified harvest was concentrated 20-fold using the Pellicon 2 mini system prior to diafiltration using a 500 mM Tris buffer at pH 8.0. Diafiltration was performed by the consistent volume method. Fresh diafiltration buffer was continuously added to the system as filtrate was permeated out of the membrane. Studies carried out using the 100 L production scale are set out in Table 5 below. The lack of fetal bovine serum in the culture medium makes is feasible to use TFF membrane partitioning diafiltration as a method of virus purification with high recovery.
TABLE 5TiterHPLC PurityRecoveryTotal Yield(vp / mL)(%)(%)(vp)Clarified Harvest1.2 × 10115.3NA1.20 × 101610-fold DF2.3 × 101278.6901.08 × 101620-fold DF2.2 × 101289.5891.07 × 101630-Fold DF2.3 × 101293.5891.06 × 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore size | aaaaa | aaaaa |
| Pore size | aaaaa | aaaaa |
| Pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com