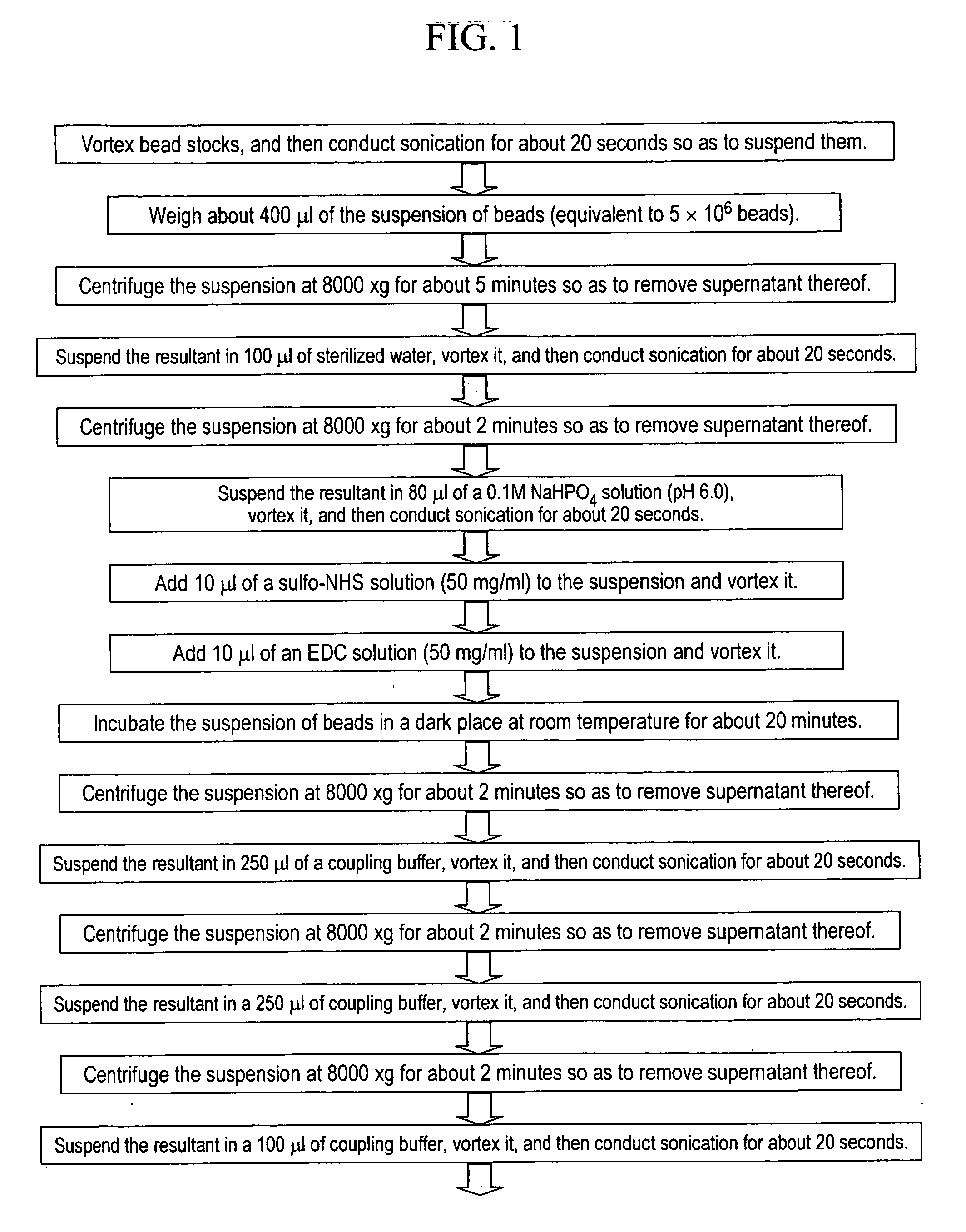
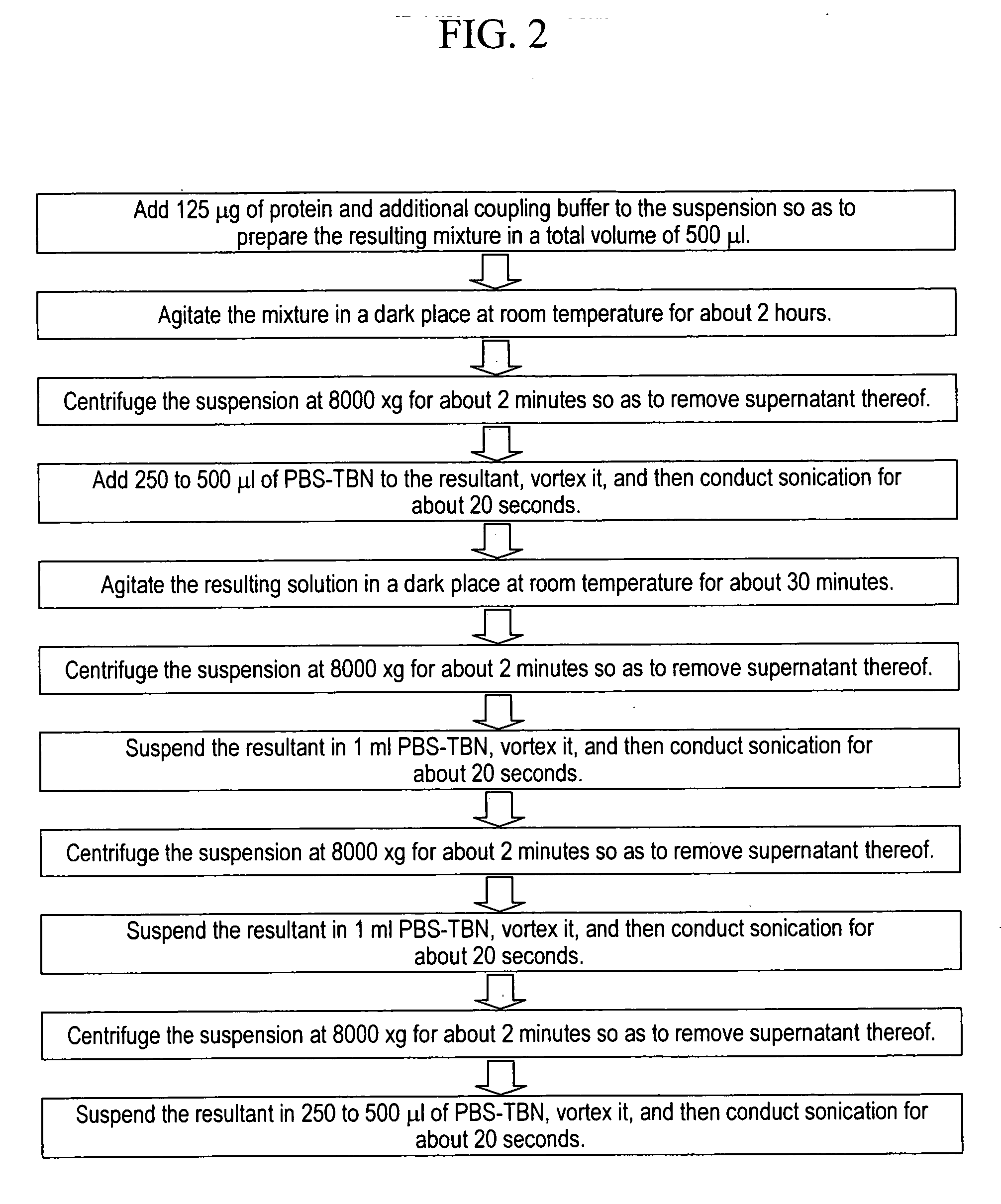
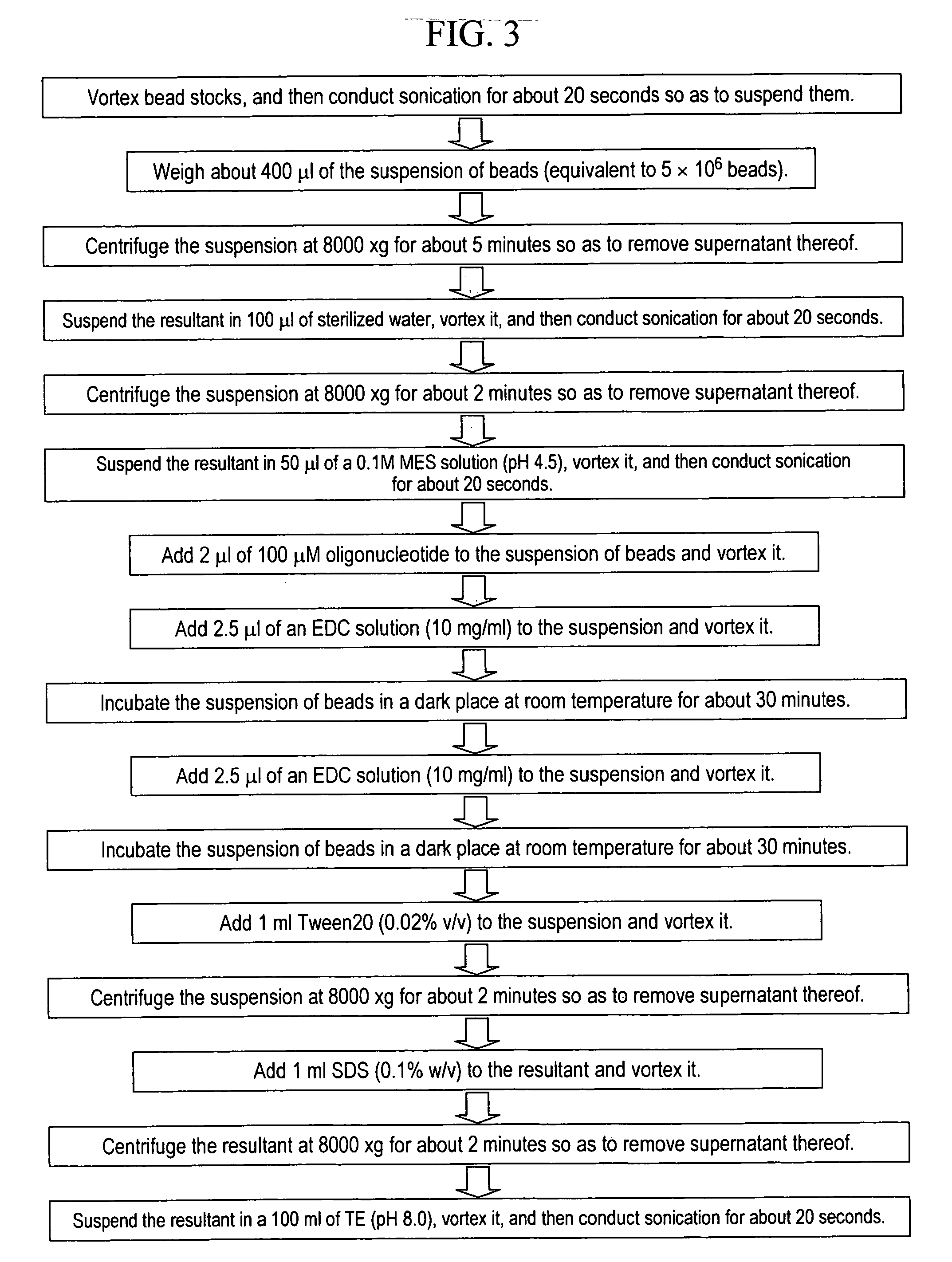
Stably preserved microspheres
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0045] A coupling reaction was performed on biotinylated oligonucleotide 20-mers, in which primary amino group had not been introduced, using microspheres activated in accordance with the present invention, as shown in FIG. 6. The results were compared with those obtained by a conventional method. Then, the side reaction was found to have been significantly suppressed compared with that in the conventional method (FIG. 8).
example 2
[0046] Nucleic acids were immobilized using microspheres produced in accordance with the present invention, as shown in FIG. 6. Conjugation of primary amino group introduced in nucleic acids with activated microspheres were performed in the various condition such as before, immediately after, and 3 days, 1 week, and 1 month after substitution using an isopropanol solvent. The experiment was carried out to confirm the presence or absence of fading of fluorescent dyes in isopropanol and the presence or absence of activity retention of active carboxylic acid ester groups (FIG. 9). The identification of bead #01 and #97 were performed ordinarily as in those without isopropanol treatment. Therefore, fluorescent dyes do not seep out during the preservation in isopropanol. Even after one month, about half of the activated ester had been maintained.
example 3
[0047] Nucleic acids were immobilized as shown in FIG. 6, using microspheres produced in accordance with the present invention. Conjugation of primary amino group introduced in nucleic acids with activated microspheres was performed in the various condition such as before, immediately after, and 3 days after substitution using an ethanol solvent. The experiment was carried out to confirm the presence or absence of fading of fluorescent dyes in ethanol and the presence or absence of activity retention of active carboxylic acid ester groups (FIG. 10). The identification of bead #6 and #85 were performed ordinarily as in those without ethanol treatment. However, about half of the activated ester had been degraded even in 3 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com