Prosthetic Grafts
a technology of prosthetic grafts and grafts, which is applied in the field of prosthetic grafts, can solve the problems of incomplete graft occlusion, synthetic or biosynthetic small vascular grafts, non-synthetic or biological, etc., and achieve the effects of enhancing angiogenesis, and enhancing patency in the prosthetic gra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0097] The following example describes the production of a prosthetic graft according to the present invention.
Primary Cell Culture:
[0098] Primary Rabbit Aortic Fibroblasts (RAF) were obtained from young male White New Zealand rabbits. The aorta was explanted, the vessel was then longitudinally opened and the endothelium was removed by gently rubbing the lumenal surface with a scraper. After this step, the vessel was cut into small pieces, it was placed in a 60 mm dish containing 2 ml of Trypsin and kept at 37° C. in a 5% CO2 incubator for 60 minutes. After this incubation, the vessel was removed and placed in 60 mm dishes with 0.5 ml of DMEM supplemented with 10% FBS containing 2 mM L-glutamine and 100 UI / mL Pen / Strep. After overnight incubation, 1.5 ml of culture medium was added. RAFs were cultured with DMEM with 10% FBS, 2 mM L-glutamine and 100 UI / mL Pen / Strep in a humidified 5% CO2 atmosphere at 37° C. Proliferating cells were used between passage 3 and 8 and were used for ...
example 2
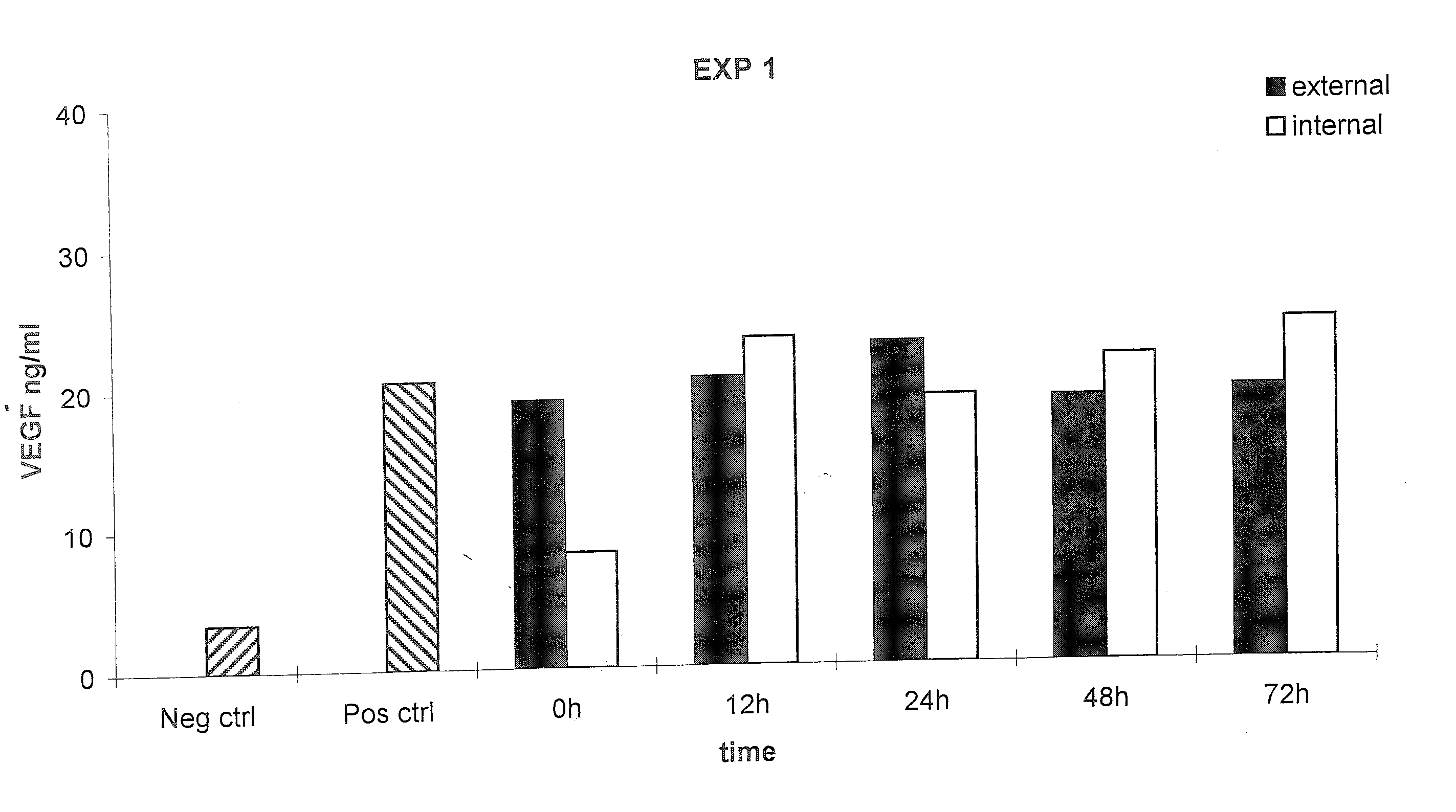
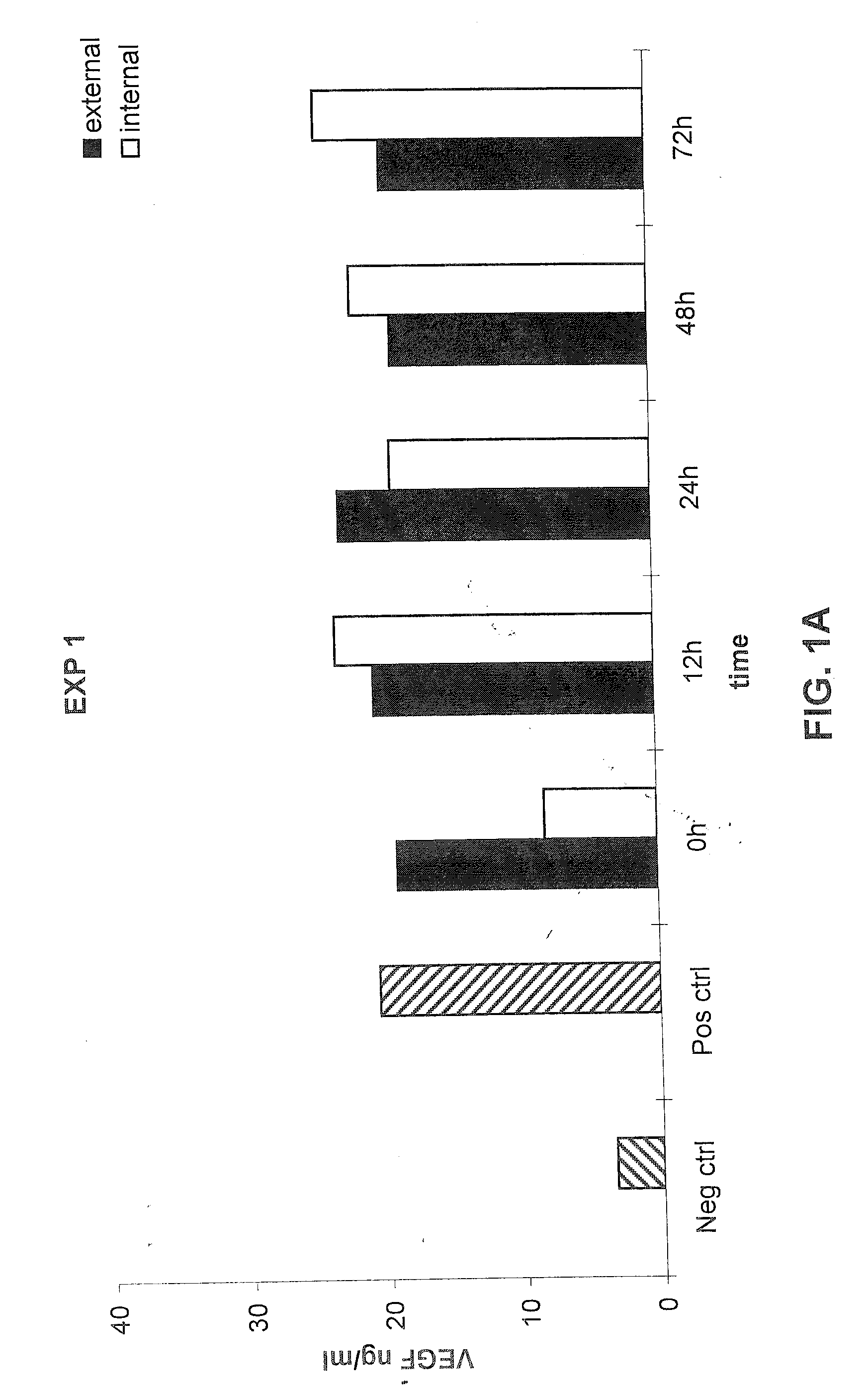
[0103] The following example demonstrates that a prosthetic graft of the present invention produces the recombinant protein both inside and outside of the graft under static in vitro culture conditions.
[0104] Production and secretion of VEGF under static in vitro conditions was measured from the PhotoFix graft seeded perivascularly with rabbit aortic fibroblasts infected with AdV cmv VEGF as described in Example 1. Briefly, the seeded PhotoFix was mounted within a closed circuit placed in a chamber and connected to a peristaltic pump. Five ml of medium in static condition were placed inside the PhotoFix, while 300 ml of medium was placed in the incubation chamber outside the PhotoFix. The chamber was incubated under static conditions under shear stress in a humidified 5% CO2 atmosphere at 37° C. At the end of incubation, external and internal medium was recovered (the internal medium was collected through a plastic outlet mounted on the circuit) and stored at −20° C. for the ELISA ...
example 3
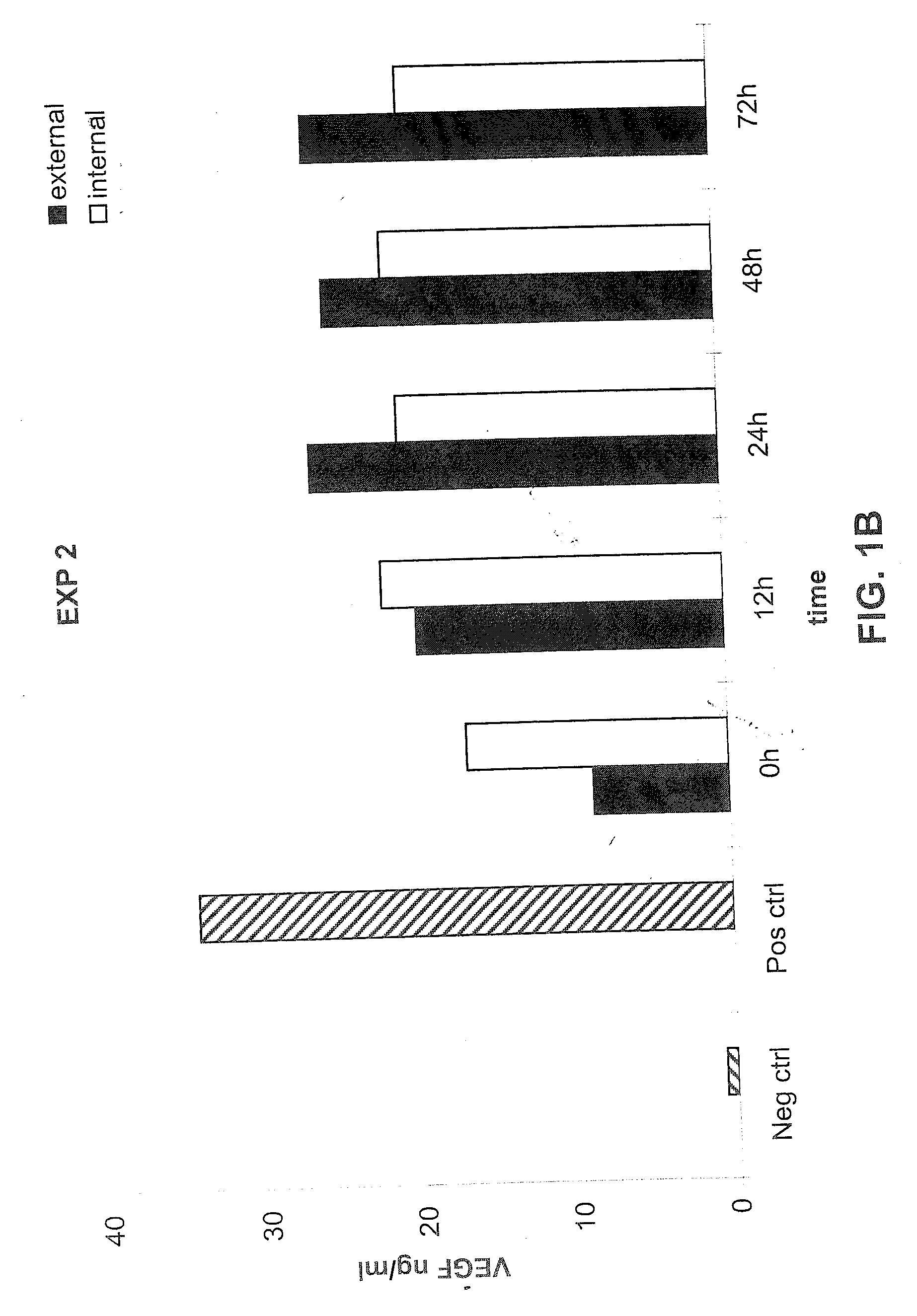
[0107] The following example demonstrates that a prosthetic graft of the present invention produces the recombinant protein both inside and outside of the graft under shear stress in vitro culture conditions.
[0108] Release of VEGF under dynamic condition-shear stress of 1.5 dyn / cm2 during in vitro conditions was measured from the PhotoFix graft seeded perivascularly with rabbit aortic fibroblasts infected with AdV cmv VEGF as described in Example 1. Briefly, as described in Example 2 above, the seeded graft was mounted within a closed circuit placed in a chamber and connected to a peristaltic pump. For the dynamic condition assays, 15 ml of medium were placed inside the PhotoFix and 300 ml of medium was placed in the incubation chamber outside the PhotoFix. The chamber was incubated in a humidified 5% CO2 atmosphere at 37° C. under dynamic conditions of shear stress by circulating the medium through the circuit at a velocity of 1.5 dyn / cm2 as indicated. The medium was collected and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| inner diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com