Sulfonated-perfluorocyclobutane polyelectrolyte membranes for fuel cells
a technology of sulfonated perfluorocyclobutane and polyelectrolyte, which is applied in the field of sulfonated perfluorocyclobutane polyelectrolyte membranes for fuel cells, can solve the problems of poor mechanical integrity and high cost, and achieve the effect of improving durability and low cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Treatment of Polymers with Structures 1, 2, and 3 with 30% Oleum.
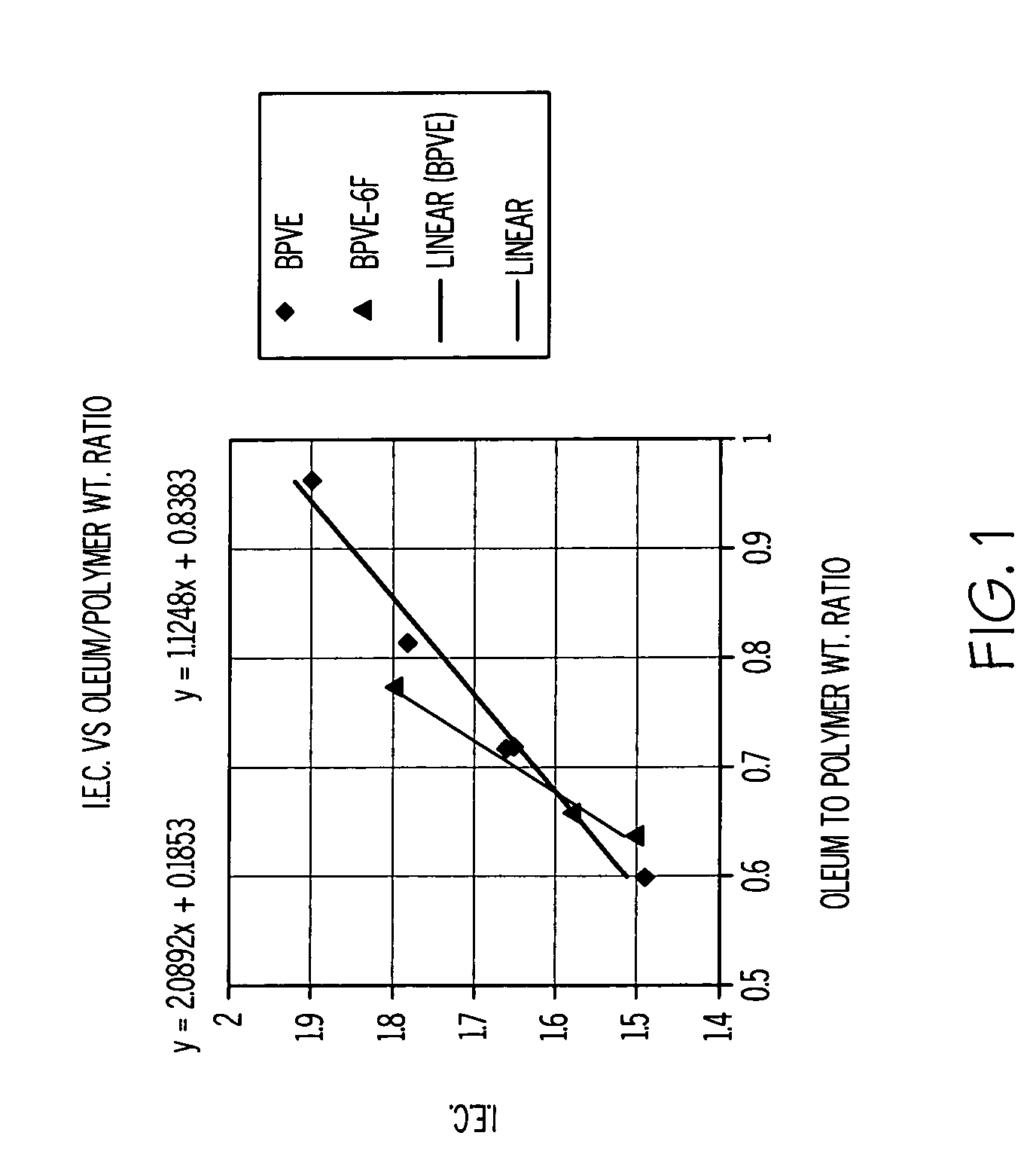
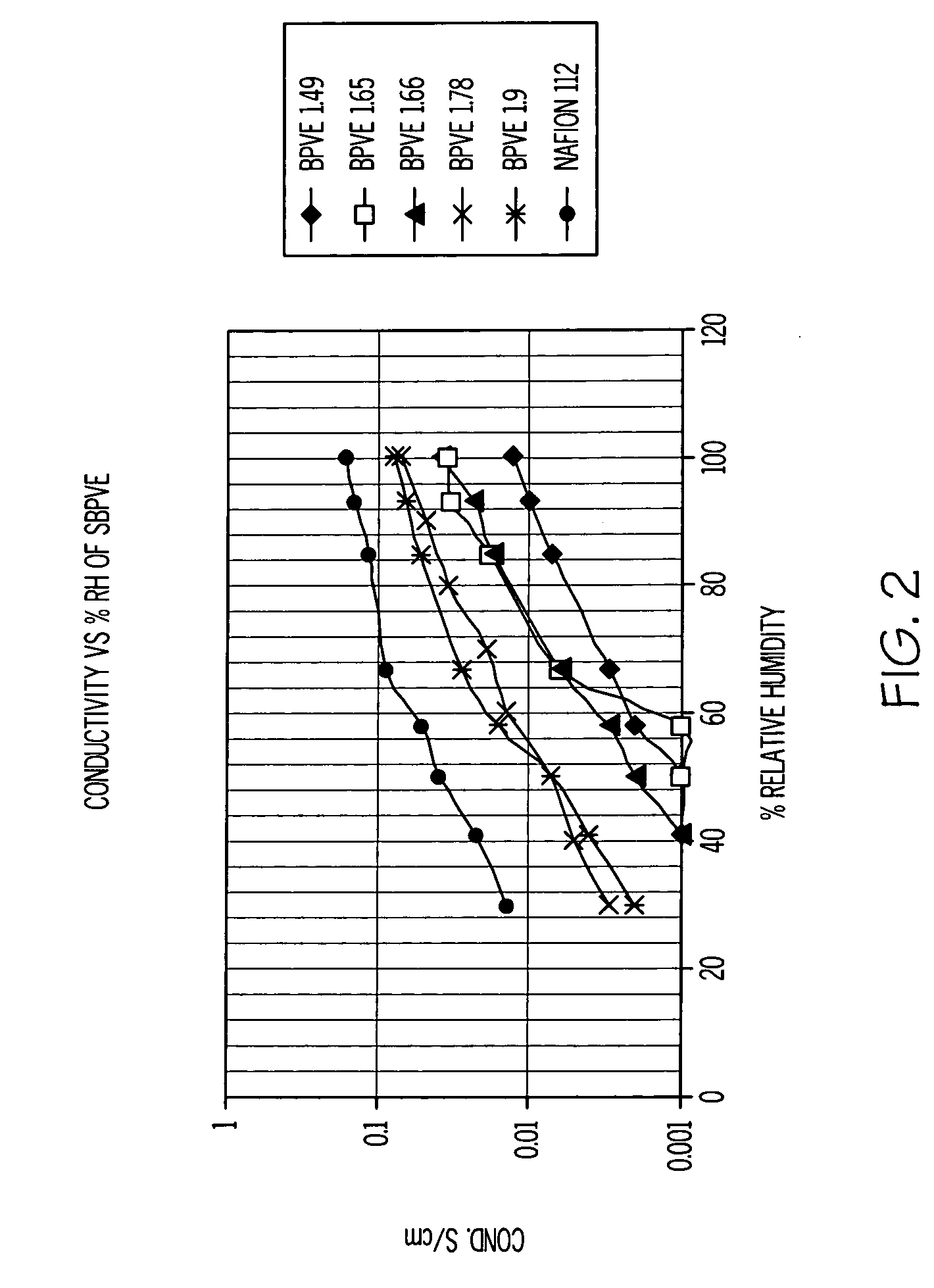
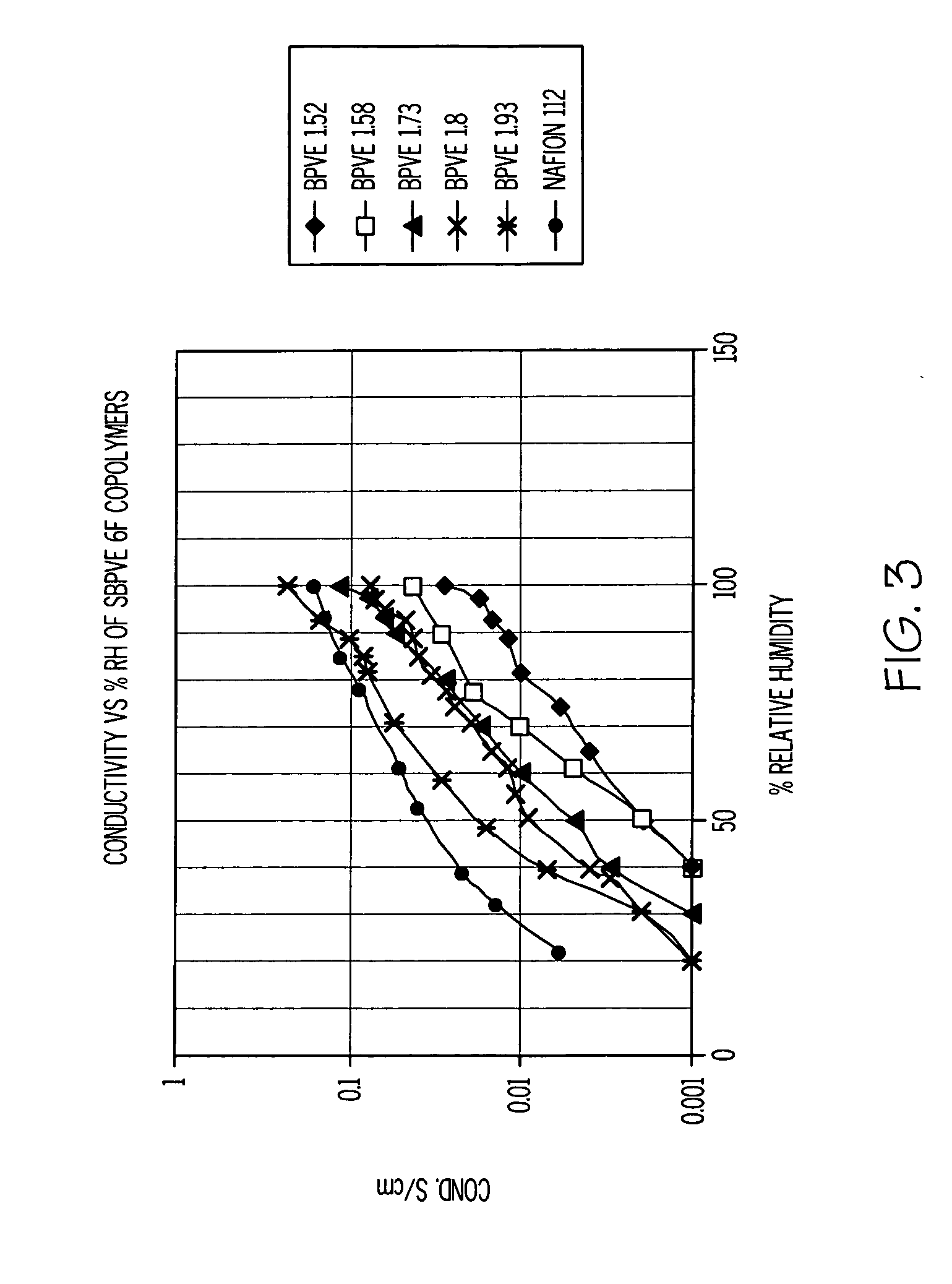
[0023] The properties of the SPFCB films are dependent on the chemical structure and the ion exchange capacity of the films, and performance can be tailored by the reaction conditions used. The amount of oleum specified in Table 1 is added to the respective polymer 1, 2 or 3, (ca 1 gram) dissolved in methylene chloride (5 mL) in a screw cap jar. The jar lid is secured and then the jar is shaken vigorously. A purple gel immediately forms, and then the jar is placed on a roll mill for between 0.5 and 1 hour. A clear liquid phase separates, which is decanted off and discarded, and the purple solid is added to vigorously stirred water (250 mL) using a Waring blender. The polymer becomes swollen white crumbs, which are isolated by vacuum filtration, washed with water and then air-dried. The sulfonated polymer (ca 1 g) readily dissolves in tetrahydrofuran (4 mL) and methanol (2 mL).
[0024] The solution is filtered through ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| electrical energy | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com