Crystal structure of a deacetylase and inhibitors thereof
a technology of crystal structure and deacetylase, which is applied in the field of crystal structure of deacetylase and its inhibitors, can solve the problems of reducing the ability of i>b. subtilis /i>to breakdown acetoin, and achieve the effect of facilitating the determination of three-dimensional structural information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Protein Production and Purification
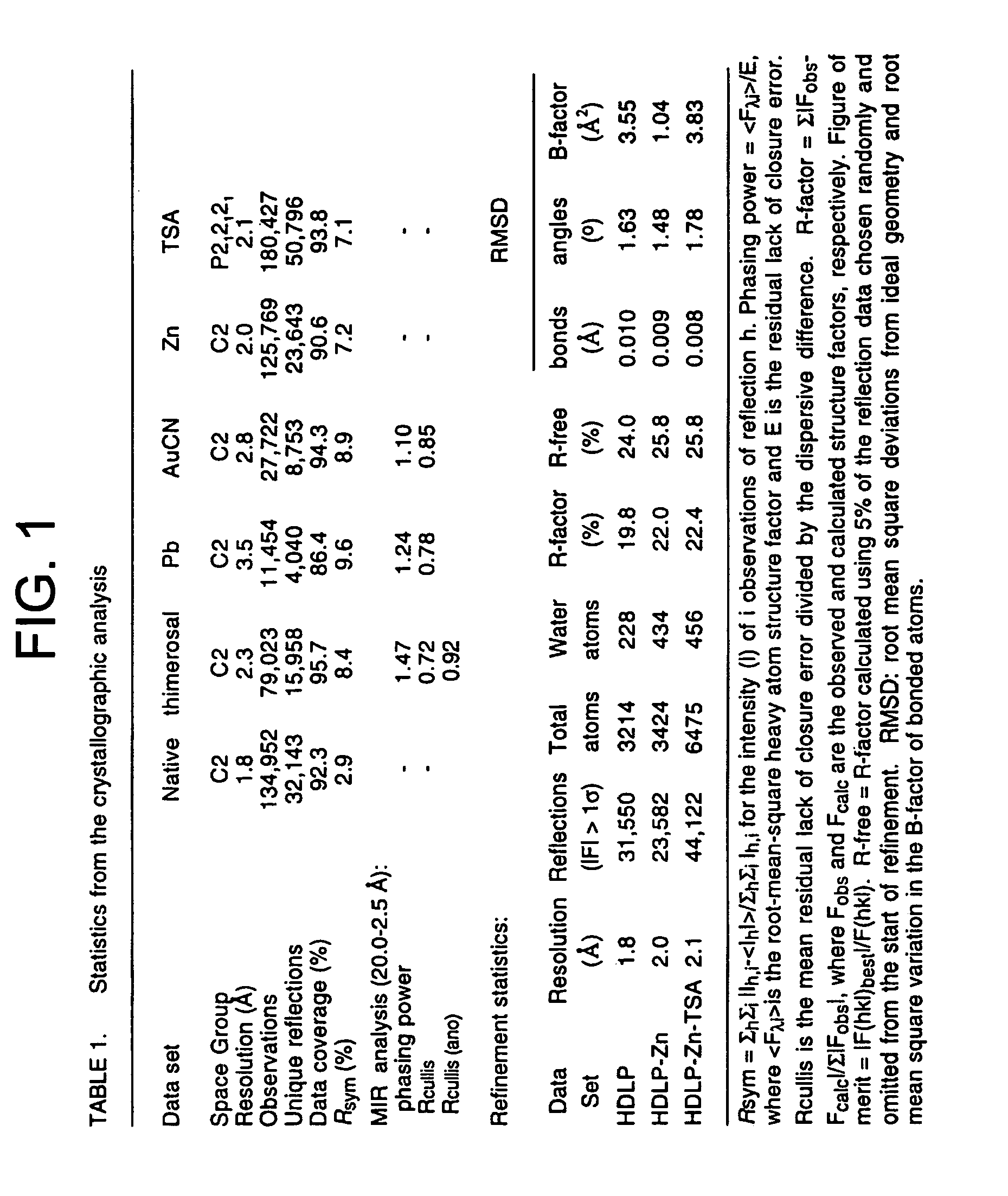
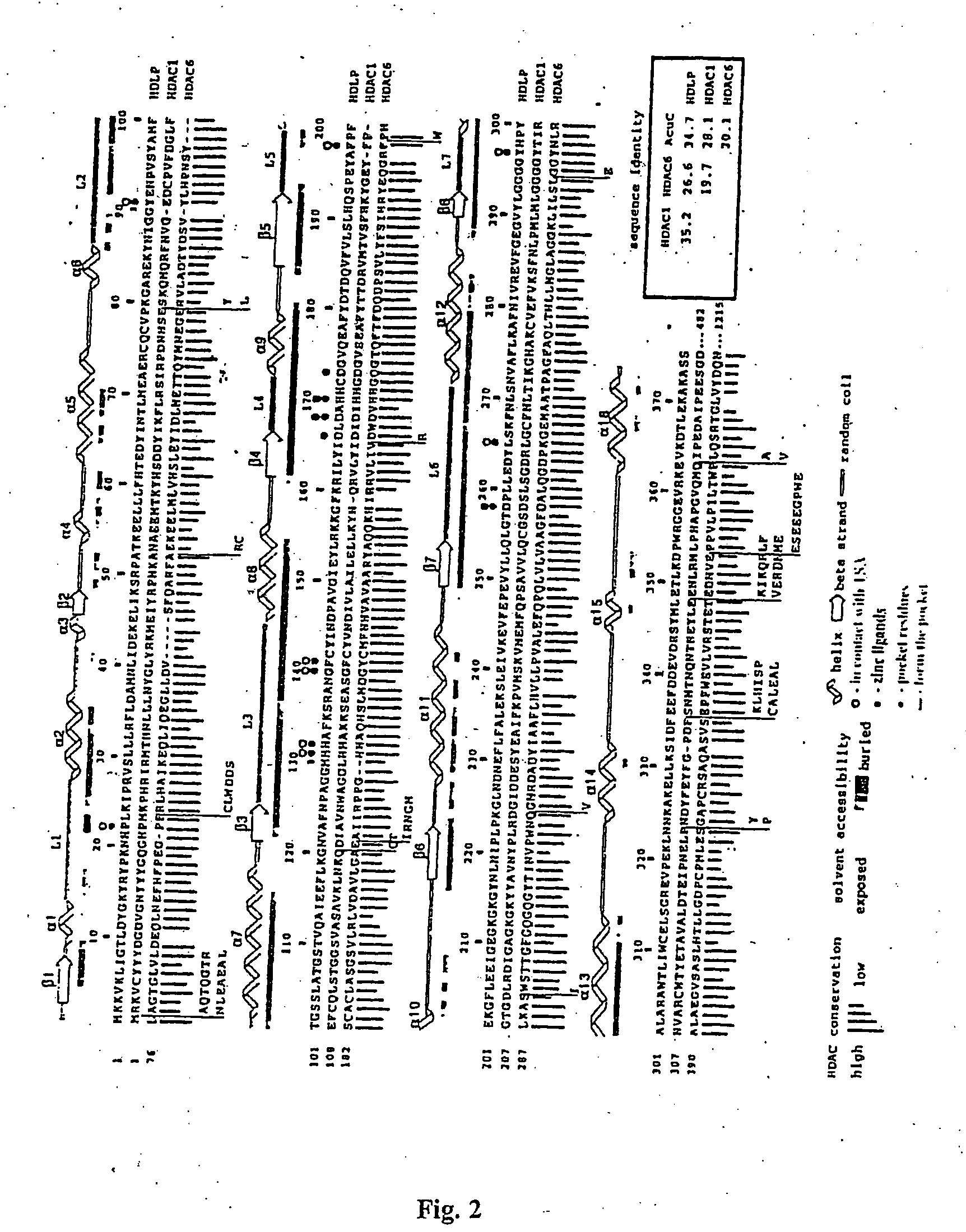
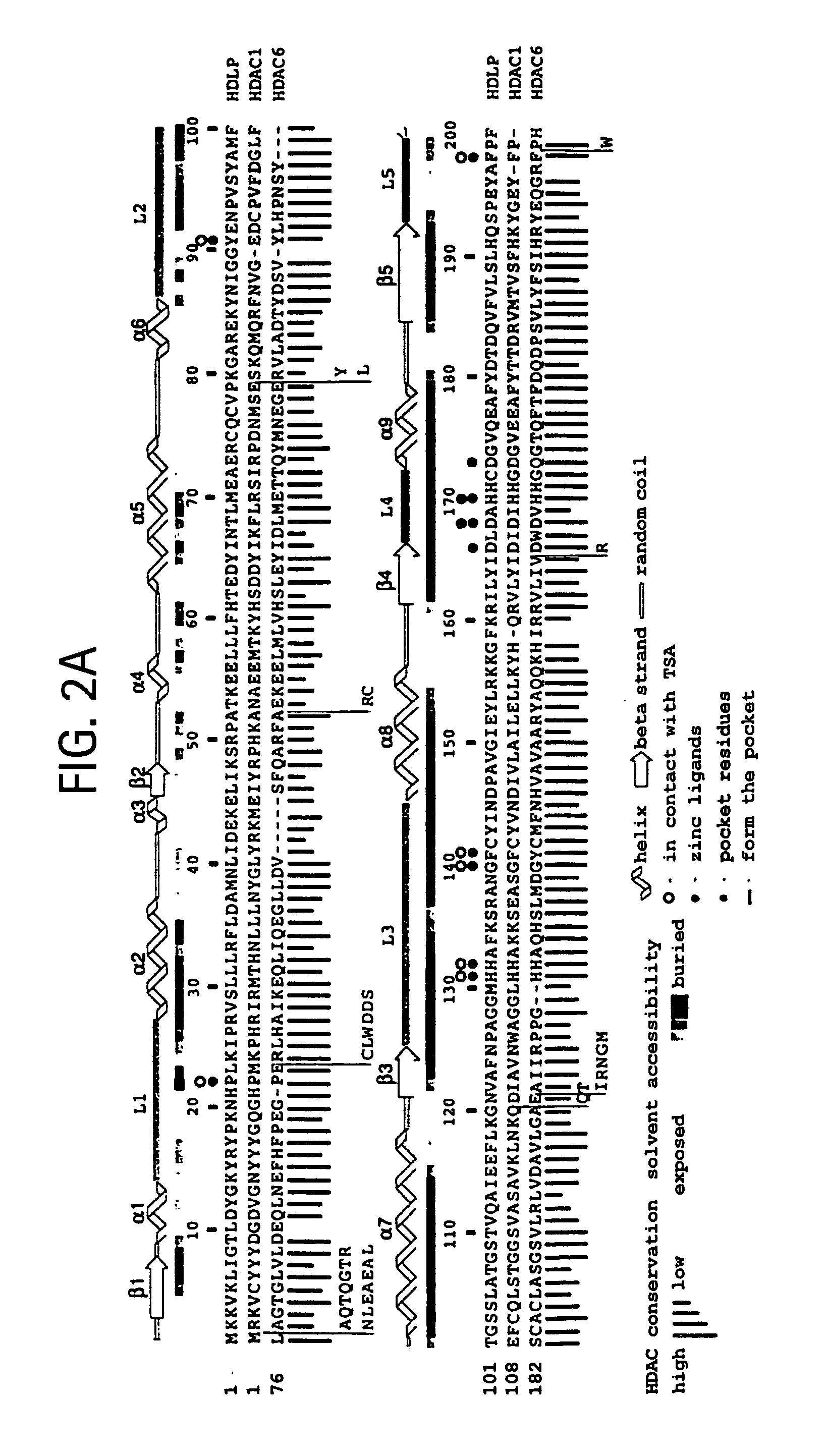
[0112] Full-length wild-type HDLP (Genbank accession number AE000719) was subcloned from an Aquifex aeolicus chromosomal DNA preparation (provided by Robert Huber of Universitaet of Regensburg, Germany) into the pGEX4T3 (Amersham-Pharmacia, Piscataway, N.J.) vector using the polymerase chain reaction (PCR). The cysteine-to-serine and active site mutants were constructed by PCR site directed mutagenesis and were sequenced. The HDLP-glutathione S-transferase (GST) fusion protein was produced in Escherichia coli, purified by affinity chromatography using a column of glutathione-sepharose resin (Amersham-Pharmacia, Piscataway, N.J.), and by anion-exchange chromatography (Q-sepharose™; Amersham-Pharmacia, Piscataway, N.J.). HDLP was cleaved from the fusion protein with thrombin at 4° C., was purified by anion-exchange (Q-sepharose™; Amersham-Pharmacia, Piscataway, N.J.) and gel filtration chromatography (Superdex™200; Amersham-Pharmacia, Piscataway, N....
example 2
Crystallization and Data Collection
[0114] Crystals of apo-HDLP were grown at room temperature by the hanging-drop vapor-diffusion method, from 7.5% isopropanol, 28% PEG 1500, 425 mM NaCl, 100 mM Tris-Cl, pH 7.0. They form in space group C2 with a 51.4 Å, b=93.8 Å, c=78.7 Å, β=96.9 Å, and contain one HDLP molecule in the asymmetric unit. Diffraction data were collected with crystals flash-frozen in a buffer of 7.5% isopropanol, 35% PEG 1500, 75 mM NaCl, 100 mM Tris-Cl, pH 8.0, at −170° C.
[0115] The structure of the HDLP-Zn2+ complex was determined from HDLP Cys75Ser / Cys77Ser double mutant crystals grown from 23% tert-butanol, 27% PEG 1500, 400 mM KCl, 100 mM bis-tris propane-Cl, pH 6.8. Space group and cell dimensions were identical to the apocrystals. The HDLP-Zn2+ crystals were harvested and frozen in 27% tert-butanol, 22% PEG 1500, 50 mM KCl, 20 mM NaCl, 0.2 mM ZnCl2, 100 mM bis-tris propane, pH 6.8, at −170° C.
[0116] Crystals of the HDLP-Zn2+-TSA complex comprised HDLP Cys75Se...
example 3
Histone Deacetylase Assays
[0120] Purified proteins were assayed by incubating 10 μg of [3H]acetyl-labeled murine erythroleukemia histone substrate and HDAC assay buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 10% glycerol) for 30-60 minutes at 37° C. in a total volume of 30 μl. The final concentrations of HDLP and HDAC1-FLAG were 3.6 μM and 0.24 μM, respectively. Assays were performed in duplicate. The reactions were stopped and the released acetate was extracted and assayed as described (Hendzel et al., 1991, J. Biol. Chem. 266:21936-21942). [3H]acetyl-labeled murine erythroleukemia histones were prepared essentially as described (Carmen et al., 1996, J. Biol. Chem. 271:15837-15844). Inhibitors were added in the absence of substrate and incubated on ice for 20 minutes, substrate was added, and the assay performed as described above. HDLP was inclubated with 20 μM ZnCl2 and 20 μM MnCl2 (H2O)4 in HDAC buffer and tested for activity.
[0121] Only HDLP dialyzed against ZnCl2 had activity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com