Novel serpentine transmembrane antigens expressed in human cancers and uses thereof
a technology of human cancer and transmembrane, which is applied in the field of new serpentine transmembrane antigens expressed in human cancers, can solve the problems of slow progress in this field, lack of effective and non-toxic systemic therapies, and still no effective treatment for metastatic prostate cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of cDNA Fragment of STEAP-1 Gene
Materials and Methods
Cell Lines and Human Tissues
[0134] All human cancer cell lines used in this study were obtained from the ATCC. All cell lines were maintained in DMEM with 10% fetal calf serum. PrEC (primary prostate epithelial cells) were obtained from Clonetics and were grown in PrEBM media supplemented with growth factors (Clonetics).
[0135] All human prostate cancer xenografts were originally provided by Charles Sawyers (UCLA) (Klein et al., 1997). LAPC-4 AD and LAPC-9 AD xenografts were routinely passaged as small tissue chunks in recipient SCID males. LAPC-4 AI and LAPC-9 AI xenografts were derived as described previously (Klein et al., 1997) and were passaged in castrated males or in female SCID mice. A benign prostatic hyperplasia tissue sample was patient-derived.
[0136] Human tissues for RNA and protein analyses were obtained from the Human Tissue Resource Center (HTRC) at the UCLA (Los Angeles, Calif.) and from QualTek, I...
example 2
Isolation of Full Length STEAP-1 Encoding cDNA
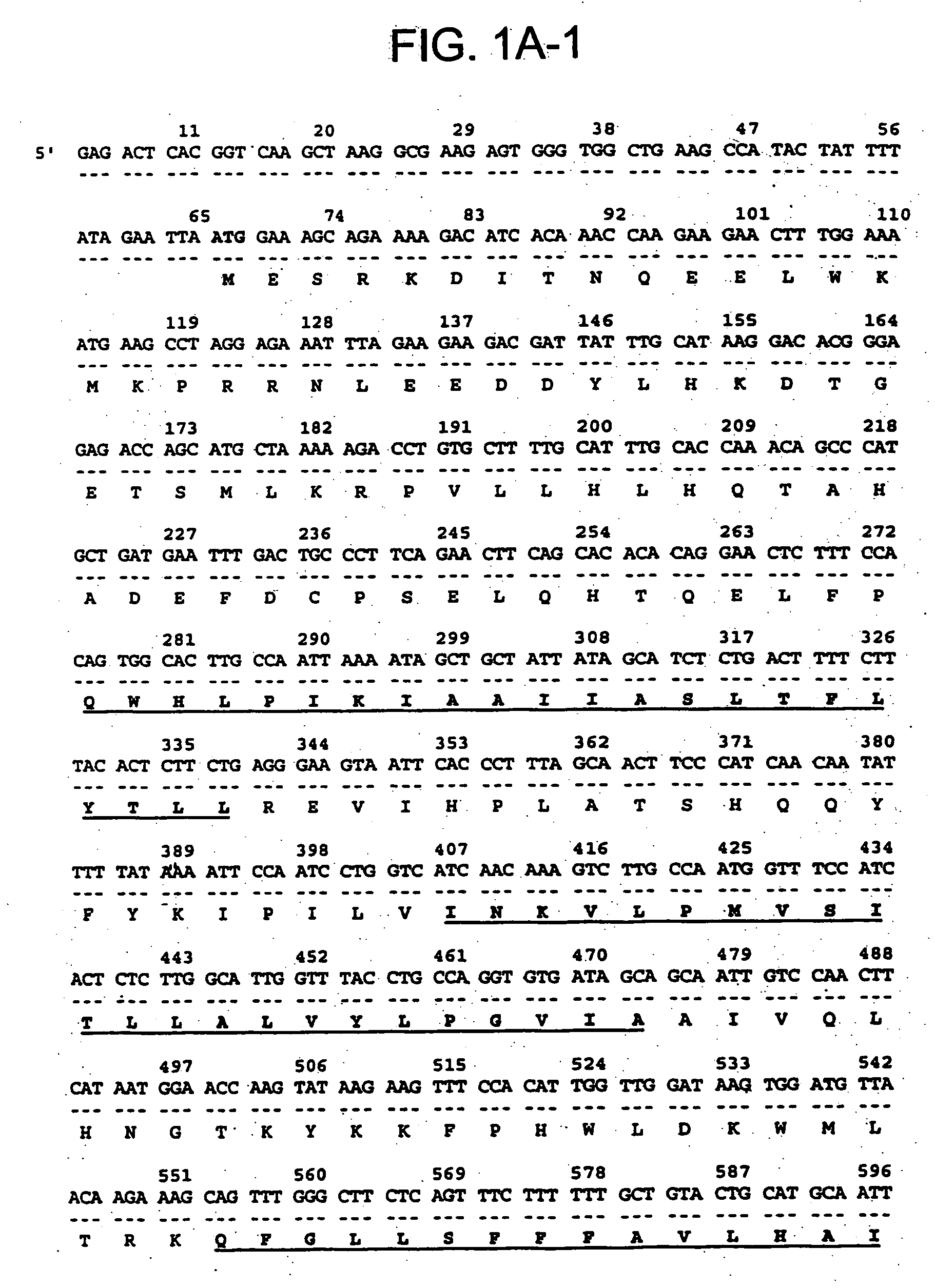
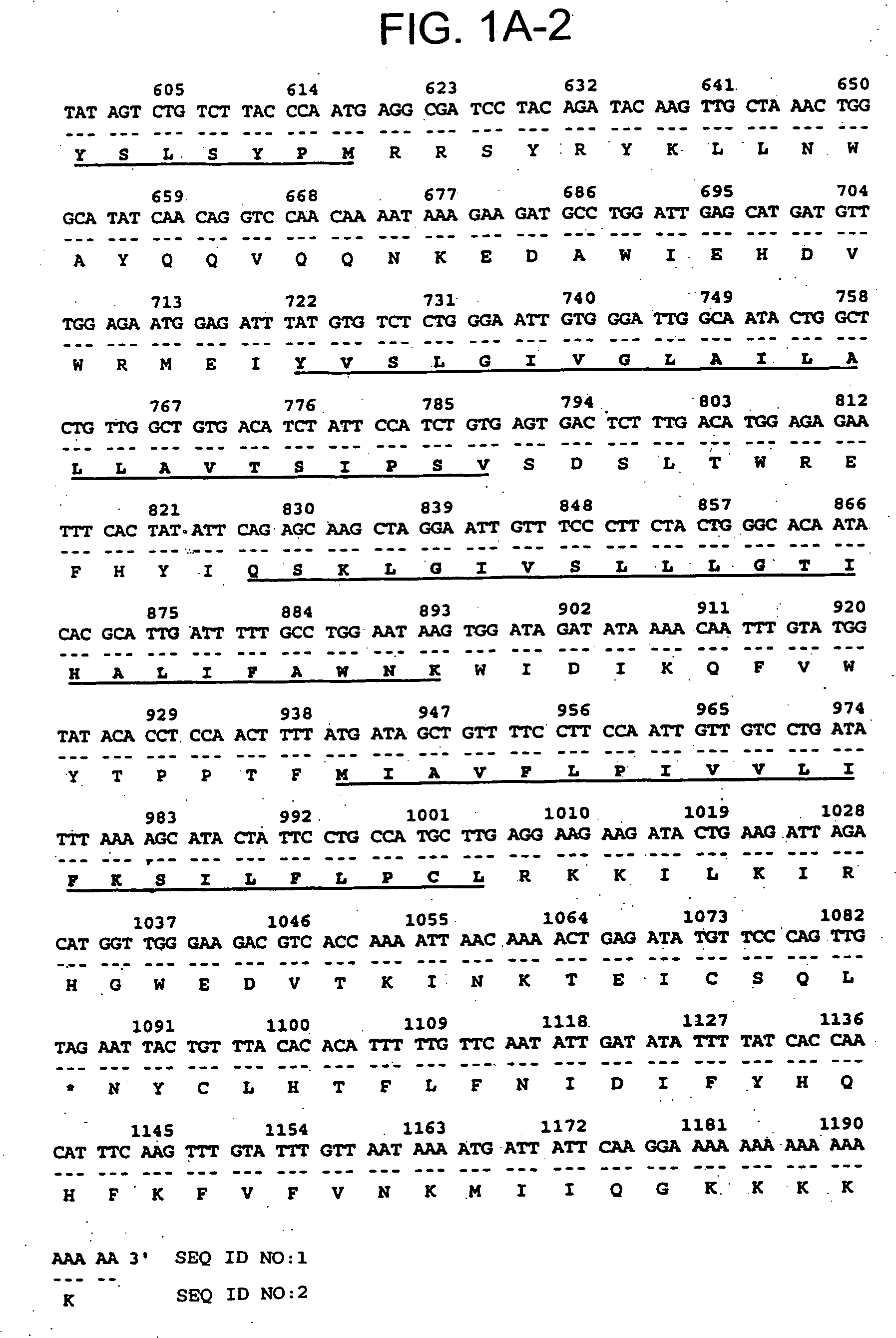
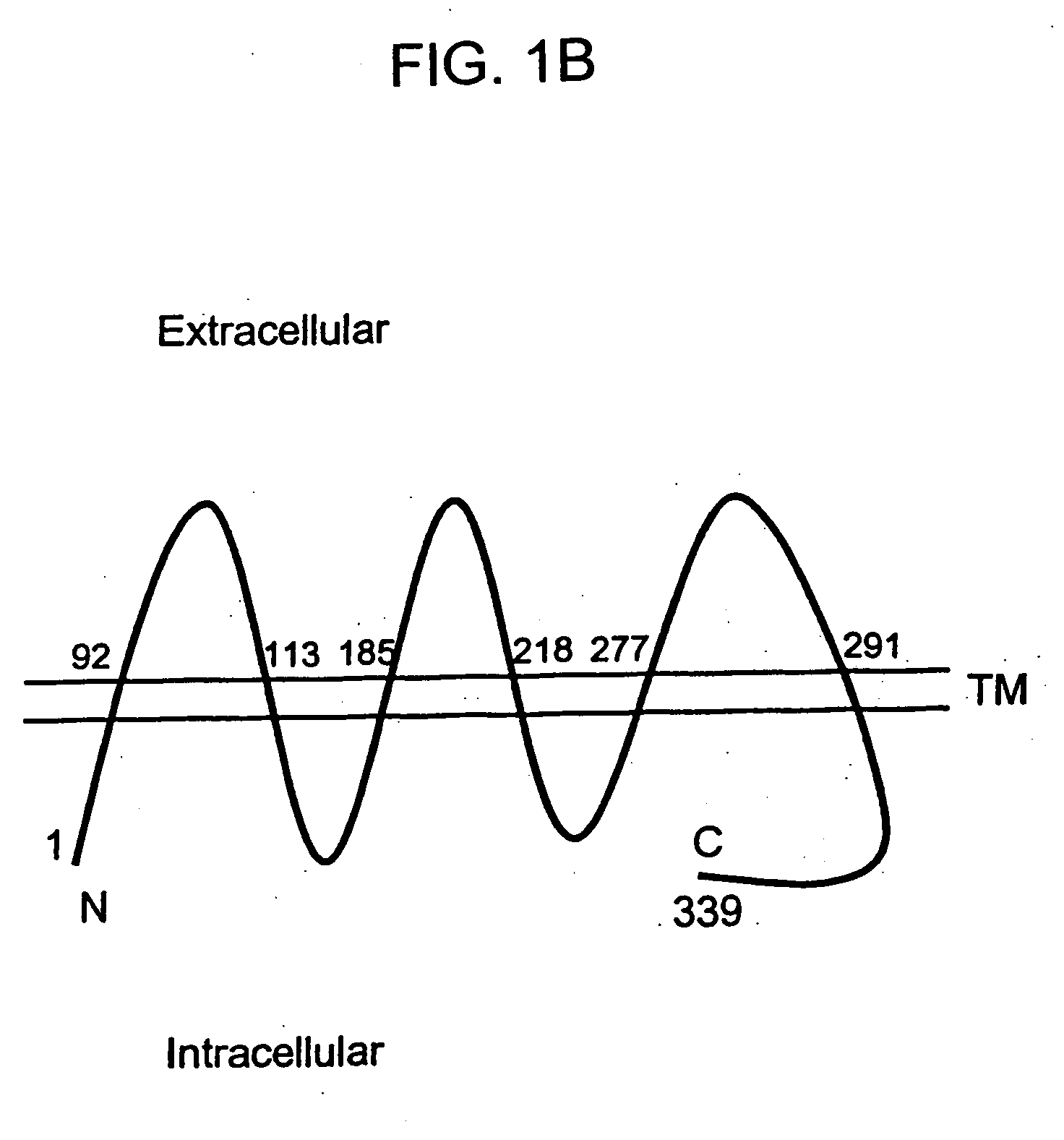
[0154] The 436 bp 8P1D4 gene fragment (Example 1) was used to isolate additional cDNAs encoding the 8P1D4 / STEAP-1 gene. Briefly, a normal human prostate cDNA library (Clontech) was screened with a labeled probe generated from the 436 bp 8P1D4 cDNA. One of the positive clones, clone 10, is 1195 bp in length and encodes a 339 amino acid protein having nucleotide and encoded amino acid sequences bearing no significant homology to any known human genes or proteins (homology to a rat Kidney Injury Protein recently described in International Application WO98 / 53071). The encoded protein contains at least 6 predicted transmembrane motifs implying a cell surface orientation (see FIG. 1A, predicted transmembrane motifs underlined). These structural features led to the designation “STEAP”, for “Six Transmembrane Epithelial Antigen of the Prostate”. Subsequent identification of additional STEAP proteins led to the re-designation of the 8P1D4 gene p...
example 3
STEAP-1 Gene and Protein Expression Analysis
[0155] In order to begin to characterize the biological characteristics of STEAP-1, an extensive evaluation of STEAP-1 mRNA and STEAP-1 protein expression across a variety of human tissue specimens was undertaken. This evaluation included Northern blot, Western blot and immunohistochemical analysis of STEAP-1 expression in a large number of normal human tissues, human prostate cancer xenografts and cell lines, and various other human cancer cell lines.
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com