Compounds, compositions and methods for the treatment of inflammatory diseases
a technology of compound composition and inflammatory disease, applied in the field of compound composition and method for the treatment of inflammatory diseases, can solve the problems of tissue injury, organ failure, inflammation in various organs of the body, etc., and achieve the effect of preventing oxidative stress-induced cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Bis- and Tris-Dihydroxyaryl Compounds of the Invention
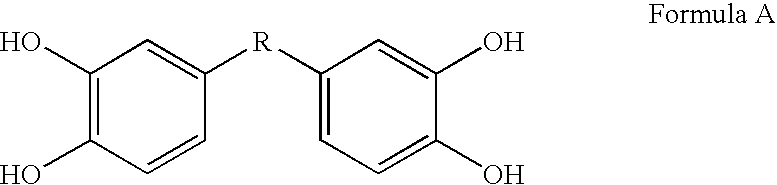
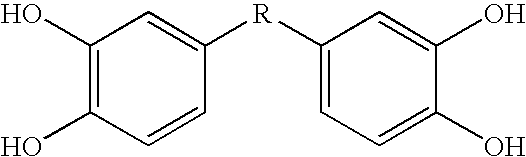
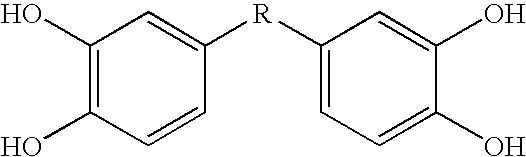
[0073] This Example describes bis- and tris(dihydroxyaryl) compounds that serve as potent inhibitors of inflammation and in particular the release of NO and TNF-α from microglial cells. A common structural motif that is present in all of the compounds disclosed herein is the presence of two or three dihydroxyaryl groups. These compounds are generally indicated on succeeding pages and identified variously herein by simple number.
example 2
Compounds of the Invention with Rigid Scaffolds
[0074] This Example illustrates six further compounds of this invention; compounds #81, 82, 83, 84, 85, and 86. These compounds have relatively rigid scaffold structures.
example 3
Methylenedioxy Analogs
[0075] A strategy for the delivery of the dihydroxyaryl compounds of this invention to improve and / or cause more favorable metabolism and bioavailability characteristics involves the protection of the hydroxy groups of the dihydroxyaryl compounds with methylenedioxy groups. This strategy is exemplified in the 80 structures shown below, and is equally applicable to protect the dihydroxyaryl groups of compounds #81-86. Methylenedioxy analogs represent intermediate hydroxy protecting structures that are made to successfully complete the synthesis of the dihydroxyaryl compounds described in the invention. These closed-ring compounds also tend to be more stable, and hydrophobic (water insoluble), and less likely to be altered or degraded due to the oxidation that could occur if hydroxyl groups were present. In addition, these compounds make good prodrugs for delivery. Hydrophobic compounds that are lipid soluble tend to be attractive compounds for delivery since th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com