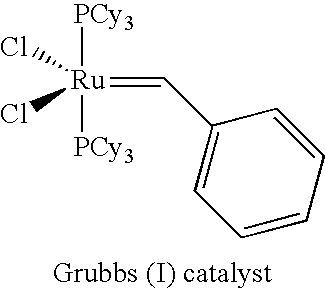
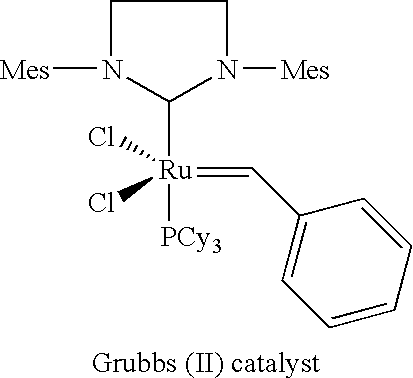
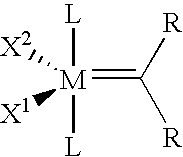Novel catalyst systems and a process for reacting chemical compounds in the presence of said catalyst systems
a catalyst system and catalyst technology, applied in the direction of physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, organic compounds/hydrides/coordination complexes, etc., can solve the problem of severe restrictions on the processing of hnbr, the inability to reduce the molar mass of the nbr feedstock used for hydrogenation, and the inability to reduce the molar mass of the nbr feedstock. to
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0211] The following experiments show that the activity of the catalyst can be increased when it is used in combination with salt additions.
[0212] The following catalysts were used for this purpose:
[0213] The Grubbs II catalyst was procured from Materia (Pasadena / California).
[0214] The Hoveyda catalyst was procured from Aldrich under the product number 569755.
[0215] The Grela catalyst was prepared by the method published in J. Org. Chem. 2004, 69, 6894-6896.
[0216] The Buchmeiser Nuyken catalyst was prepared as described in Chemistry European Journal 2004, 10(3), 777-785.
General Method for the Metathetic Degradation of Nitrile Rubber (“NBR”)
[0217] The degradation reactions described below in the trials 1 to 6 were carried out using the nitrile rubber Perbunan® NT 3435 from Lanxess Deutschland GmbH. This nitrile rubber had the following characteristic properties:
Acrylonitrile content:35% by weightMooney viscosity (ML 1 + 4 @100° C.):34 Mooney unitsResidual moisture conte...
example 7
Use of LiBr for the Ring-Closing Metathesis of Diethyl Diallylmalonate
[0258] The ring-closing metathesis of diethyl diallylmalonate was carried out once without and once with 1 mg of LiBr (Examples 7.01 and 7.02) and also once without and once with 1 mg of CsBr (Examples 8.01 and 8.02).
[0259] To carry out the experiments, 10 mg of Grubbs II catalyst were in each case placed in an NMR tube. In the examples according to the invention, which were carried out with additions of LiBr (Example 7.02) or CsBr (Example 8.02), 1 mg of LiBr or 1 mg of CsBr were weighed into the NMR tube in addition to the Grubbs II catalyst (10 mg). Subsequently, firstly 0.3 ml of chlorobenzene and then 0.2 ml of CDCl3 were added at room temperature by means of a syringe. The contents of the NMR tube were mixed by shaking. After 2 minutes in each case, 0.15 ml of diethyl diallylmalonate was added by means of a syringe. The reaction conditions were determined by means of 1H-NMR spectroscopy at room temperature...
example 8
Use of CsBr for the Ring-Closing Metathesis of Diethyl Diallylmalonate
[0261] The experiments were carried out in a manner analogous to Example 7 using 1 mg of CsBr instead of 1 mg of LiBr.
With salt addition (8.02)Without salt addition (8.01)1 mg of CsBrTime [min.]Conversion [%]Conversion [%]0001513.416.53025.340.36046.568.99071.984.715096.2100
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| polydispersity index PDI | aaaaa | aaaaa |
| polydispersity index PDI | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com