Method of Inhibiting Secretase Activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0093] This example is a working example of Herp screening using the Yeast Two Hybrid system.
[0094] A MATCHMAKER system (CLONTECH) was used for the Yeast Two Hybrid system, which employed the yeast transformation method (Pro. Natl. Aca. Sci. USA, 88: 9578-9582, 1991).
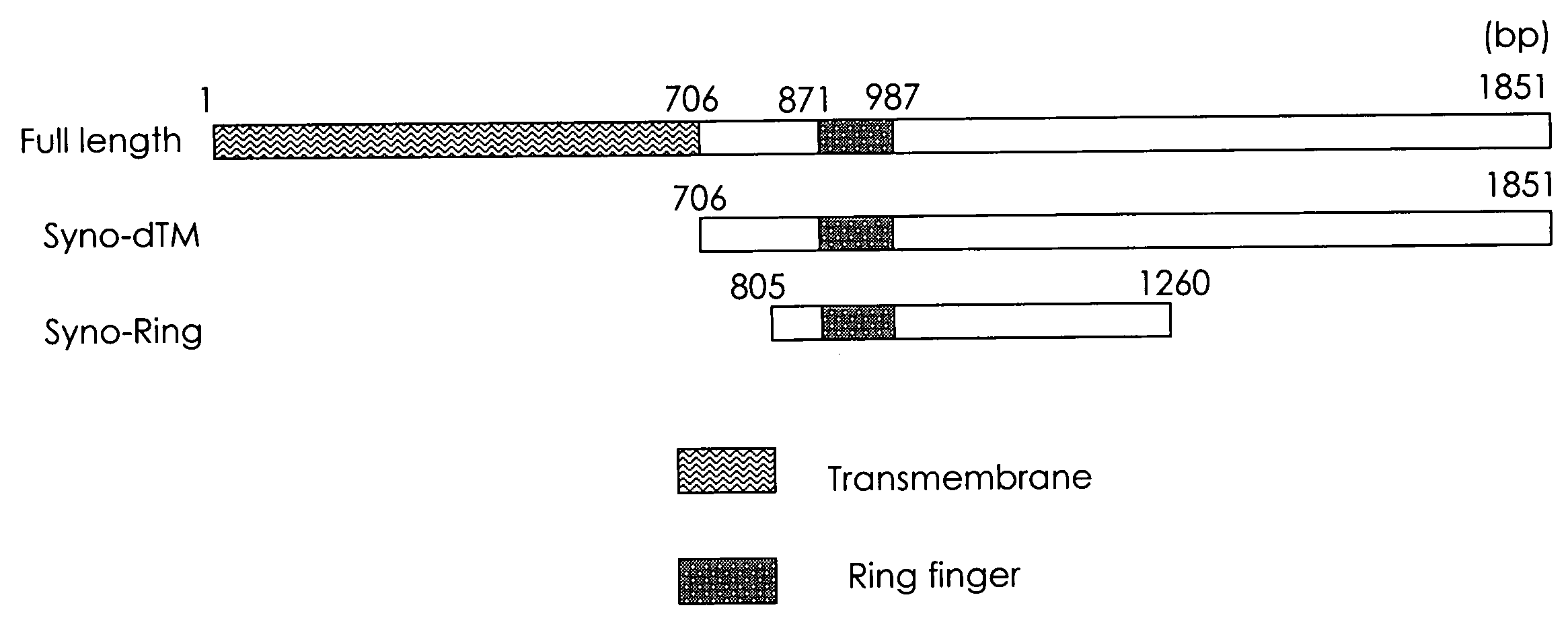
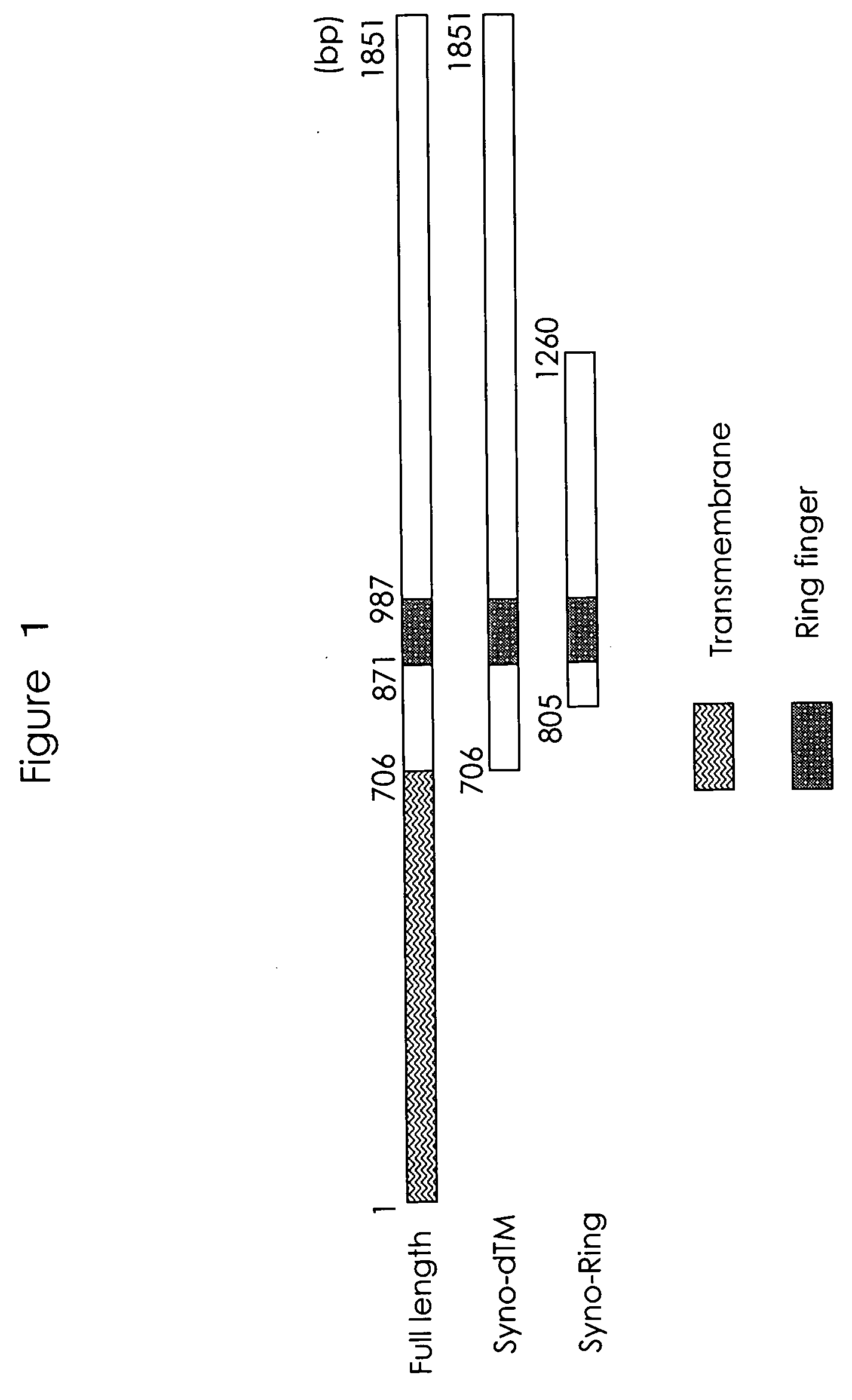
[0095] The end of synoviolin cDNA from 706 bp (amino acid #236) to 1851 bp (amino acid #617) or from 805 bp (amino acid #269) to 1260 bp (amino acid #420) was inserted into the Eco R I / Xho I site of a pGBT9 vector as EcoR I / Xho I (from 706 bp (amino acid #236) to 1851 bp (amino acid #617) of synoviolin is hereinafter called Syno dTM, while from 805 bp (amino acid #269) to 1260 bp (amino acid #420) is called Syno Ring: see FIG. 1). In FIG. 1, “bp” indicates a position in the nucleotide sequence.
[0096] The library used for Herp screening consisted of human cartilage-derived cDNA inserted into a pACT2 vector (pACT2-Y). Following 15 minutes of heat shock at 42° C., pGBT9-Syno dTM or pGBT9-Syno Ring (2.0 μg) were inserted...
example 2
[0100] In this example, synoviolin was bound to Herp in vitro.
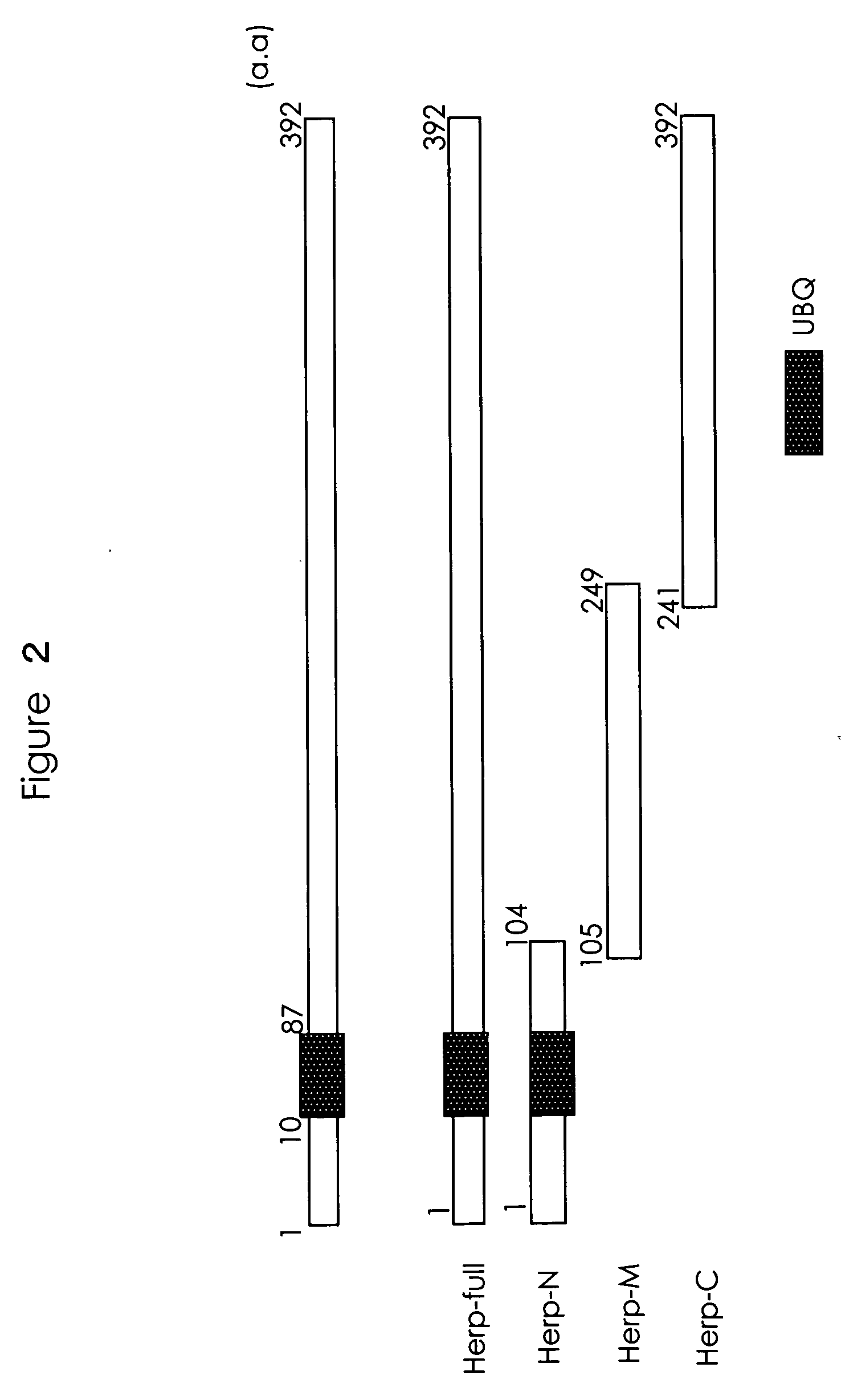
[0101] pcDNA3-Flag-Syno dTM was prepared with synoviolin from 706 bp to 1854 bp and a TNT-coupled Translation System (Promega) inserted, and subjected to in vitro translation to prepare (i) a Flag-Syno dTM fusion protein, (ii) a Herp GST fusion protein, and (iii) a GST fusion protein with fragmented Herp (FIG. 2). In FIG. 2, “a.a.” shows the position in the amino acid sequence, while UBQ indicates a ubiquitin domain.
[0102] These proteins and (iv) GST protein as a control were added to a binding buffer comprising 20 mM Hepes (pH 7.9), 100 mM NaCl, 1 mM EDTA, 0.05% Tween, 5% Glycerol, 1 mM DTT, 0.2 mM NaVO4, 5 mM NaF and 1 mM PMSF, and reacted for 16 hours at 4° C. The reaction product was subjected to SDS-PAGE, and radioactivity was detected with an image analyzer (BAS2000, Fujix).
[0103] As a result, binding was observed between the Herp-M and Herp-C GST fusion proteins and the [35S]pcDNA3-FlagSyno dTM.
[0104] No bindin...
example 3
[0105] In this example, synoviolin and Herp were bound in vivo.
[0106] 2×106 HEK293 cells were inoculated on a 10 cm dish and cultured for 24 hours, and 3 μg each of pcDNA3-HA / Syno with synoviolin from 1 bp to 1854 bp inserted and pcDNA3-Flag / Herp with Herp from 1 bp to 1176 bp inserted were introduced into the cells. 24 hours after gene introduction the cells were collected and lysed with lysis buffer, and immunoprecipitation was performed overnight at 4° C. with anti-HA and anti-Flag antibodies. The bound proteins and free proteins were separated by centrifugation, and detected by Western blotting. Anti-HA and anti-Flag antibodies were used for detection.
[0107] As a result, a Flag-Herp band was confirmed by detection with anti-Flag antibodies following immunoprecipitation with anti-HA antibodies in the cells co-expressing synoviolin and Herp. Moreover, an HA-synoviolin band was confirmed by detection with anti-HA antibodies following immunoprecipitation using anti-Flag antibodies...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com