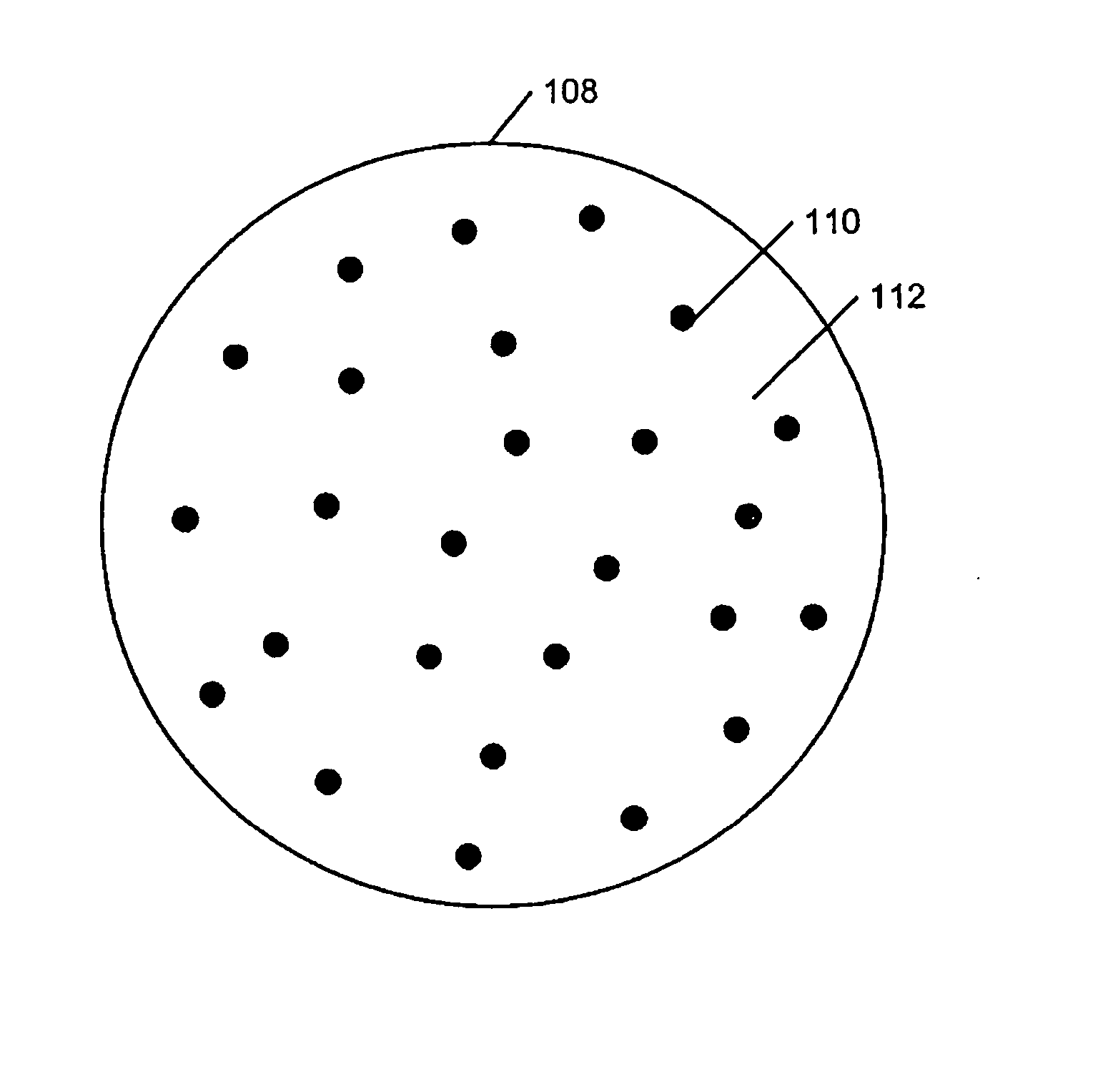
Gas dispersion manufacture of nanoparticulates, and nanoparticulate-containing products and processing thereof
a technology of nanoparticulate and gas dispersion, which is applied in the field of nanoparticulate materials, can solve the problems of forming, processing, transporting and using nanoparticulates, affecting and not providing the same advantages. it can achieve the effect of promoting the formation of nanoparticulate materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Iron Oxide Nanoparticulates and Salt Matrix
[0163] Several precursor mediums are prepared containing: Fe(NO3)3.9H2O, as a precursor to iron oxide nanoparticulates; a salt, NaNO3 or NaCl, as a precursor to a matrix material and deionized water. The precursor mediums are processed into a powder containing multi-phase particles including iron oxide nanoparticulates in a salt matrix, which is dissolved away in a subsequent step. The weight ratio of Fe(NO3)3.9H2O to salt in the precursor mediums is varied between 1:2 and 1:8, based on the final amount of iron oxide desired in the multi-phase particles. The precursor mediums are processed using a spray pyrolysis system that generates droplets of the precursor mediums, using an ultrasonic generator, and heats the droplets in a tubular hot wall reactor at temperatures ranging from 400° C. to 1000° C. The processing conditions for the production of the powders containing the multi-phase particles are summarized in Table 14.
[0164] Thermograv...
example 2
Phosphor Nanoparticulates and Salt Matrix
[0179] Precursor mediums are prepared for making powders containing multi-phase particles with phosphor nanoparticulates, having the general composition Na(Y,Yb,Er)F4 nominally including 69 mol % Y, 30 mol % Yb and 1 mol % Er. The following precursors are included in the precursor mediums: Y2(CO3)2.xH2O, Er2(CO3)3.xH2O, Yb2(CO3)3.xH2O and NaHCO3. As a first step to the preparation of the precursor mediums, HOfAc is added to the carbonate precursors to form the corresponding metal trifluoracetates. Complete reaction of the precursors to the metal trifluoracetates is indicated by the presence of a clear precursor solution. A matrix precursor is then dissolved in the clear precursor solution. NaNO3, NaF or NaCl are dissolved in the precursor medium as precursors for a salt matrix. The different precursor mediums prepared are listed in Table 15. Each precursor medium also includes an excess of HOfAc.
TABLE 15Precursor MediumsPhosphorMatrixPrecu...
example 3
Yttria Nanoparticulates and Salt Matrix
[0192] A precursor medium containing yttrium nitrate hexahydrate, Y(NO3)36H2O and sodium nitrate NaNO3, dissolved in deionized water is prepared. The precursor medium contains:
Y(NO3)3*6H2O85gNaNO3101.9gH2O200g
[0193] The precursor medium is sprayed out of a pump spray bottle onto a hotplate. The hotplate has a sheet of foil and a glass plate, onto which the precursor medium is sprayed. The glass plate is at a temperature of 300° C. The resulting deposit is scraped off of the glass plate and is then heated in a porcelain crucible using a box furnace. The box furnace is operated by being ramped up at 15° C. / minute to a temperature of 550° C. and held at 550° C. for 12 minutes, then cooled at about 10° C. / minute.
[0194] After processing the powder, examination indicates that the salt matrix melted during heating in the furnace. Also, some of the salt matrix vaporizes during the thermal treatment in the furnace, but the amount is not quantified. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| weight average size | aaaaa | aaaaa |
| weight average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com