Prevention of allergy in children
a technology for allergy prevention and children, applied in the field of allergy prevention in children, can solve the problems of ineffective measures, increased risk of hypersensitivity or inflammation, and unknown, and achieve the effect of reducing the chance of affecting the intinal mucosa and prolonging the time span
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Characterization of CD25+ Tregs
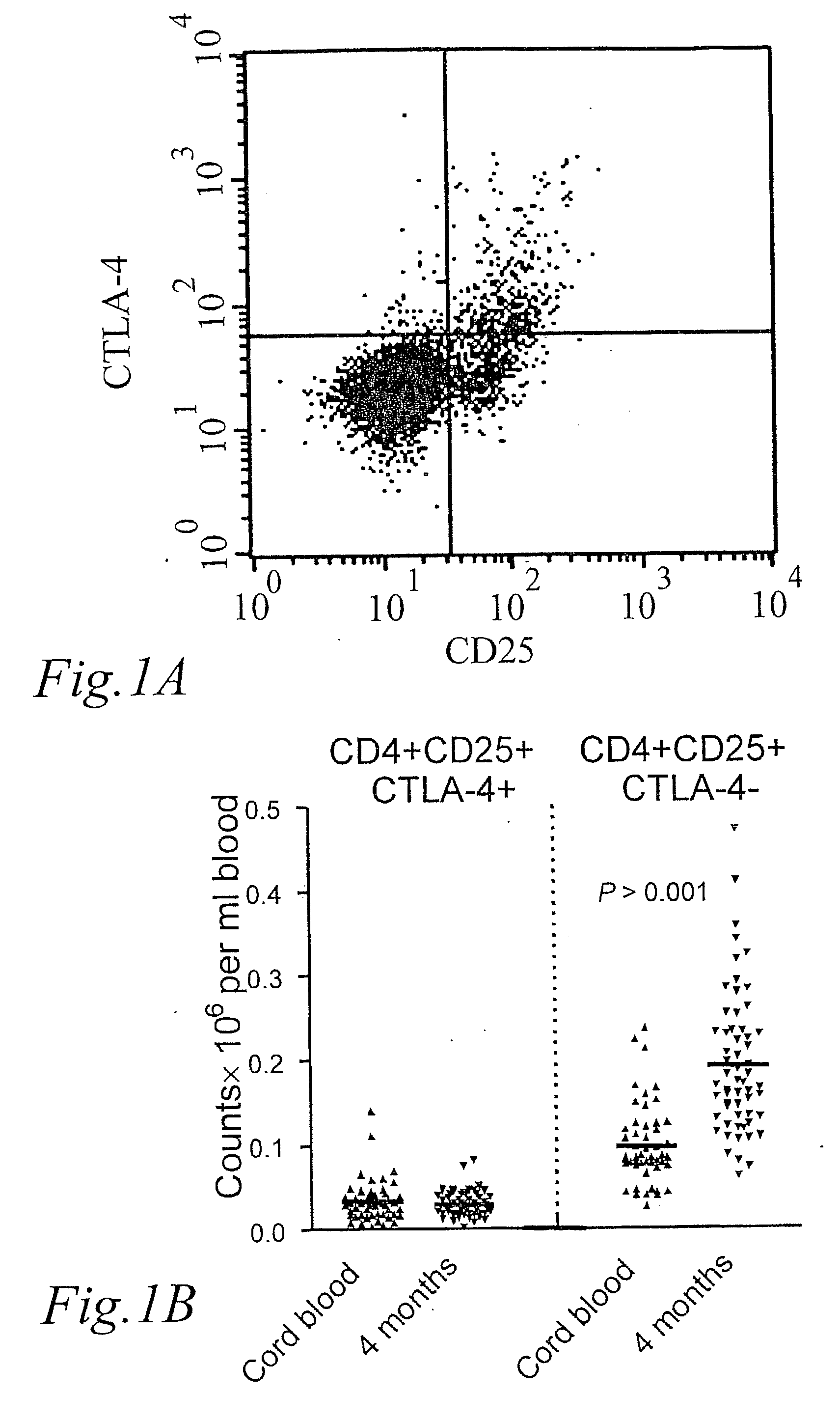
[0040] The development of CD25+ Tregs during the first 4 months of life were characterized by analysing the number of cells expressing surface CD4 and CD25 and intracellular CTLA-4 in peripheral blood obtained at 4 months of age compared to cord blood. FIG. 1A shows a dot-plot of the expression of CD25 and CTLA-4 on gated CD4+ T-cells. The cells in the upper right quadrant (A) were presumed to be CD25+ Tregs.
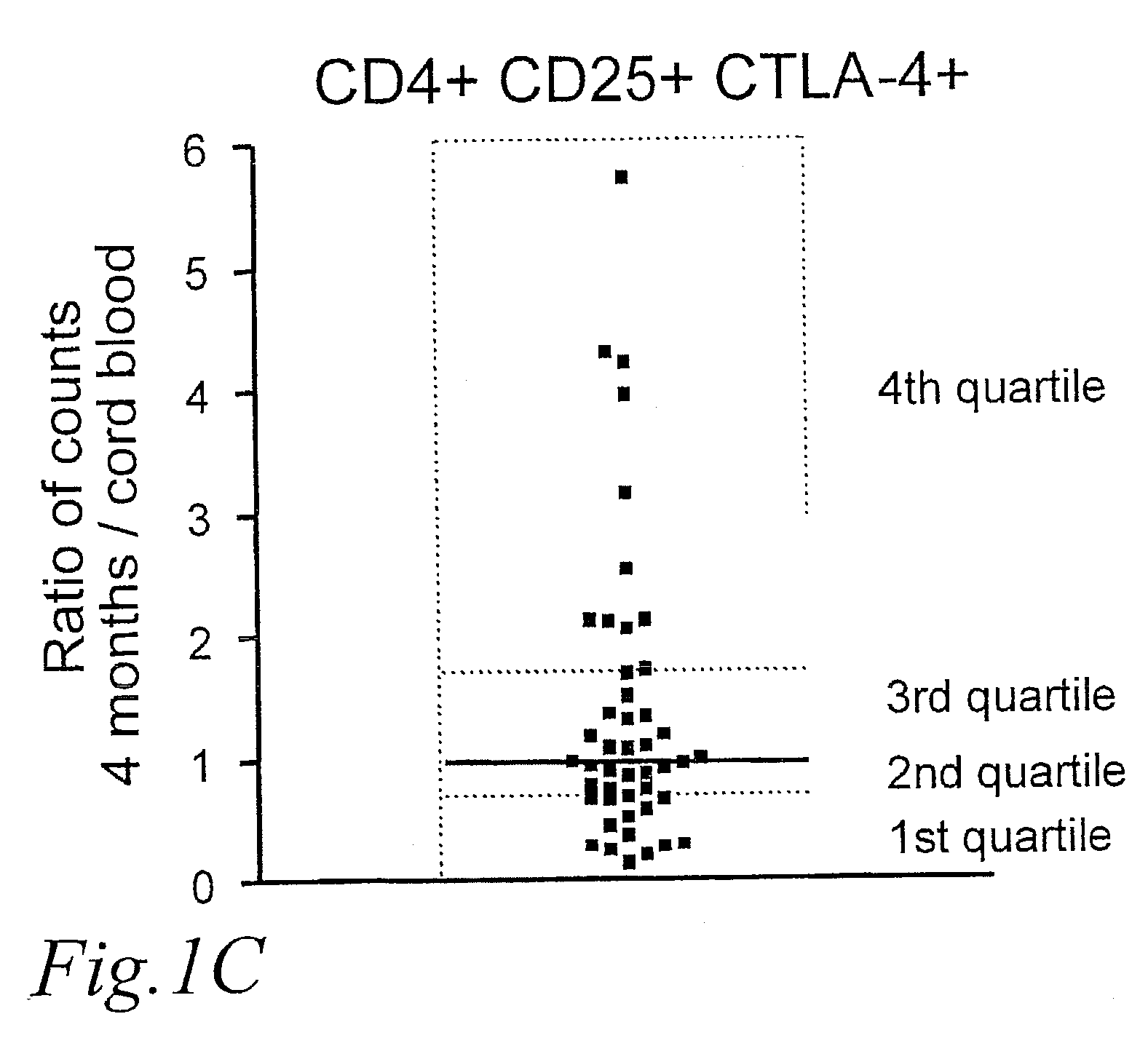
[0041] However, CD25 may also be upregulated on T-cells during activation, and whereas the average number of CD25+ Tregs was similar in newborns and children 4 months of age, the number of CD4+CD25+ CTLA-4− increased with age, indicating that they represented activated T-cells. This is seen in FIG. 1B, which shows the number of CD25+ Tregs; CD4+CD25+CTLA-4+ and CD4+CD25+CTLA-4− cells×10−6 per ml blood, respectively, in newborns and children at 4 months of age. CD4+CD25+ CTLA-4− cells are cells that recently have been activated, expressing CD25 on...
example 2
Intestinal Colonization by Toxin-Producing S. aureus Induces Expansion of Cd25+ Tregs
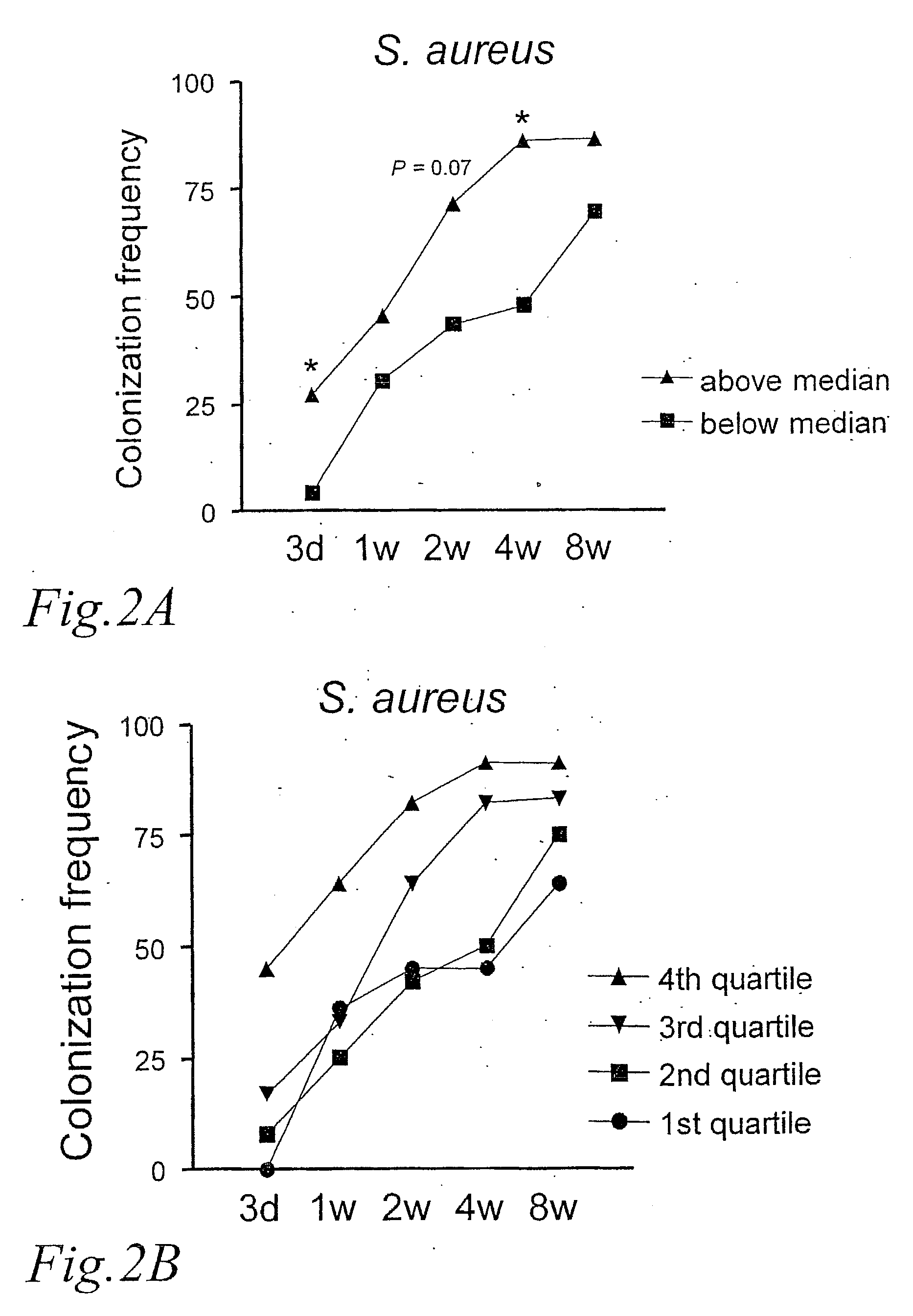
[0043] The type of colonization pattern was examined in relation to the degree of CD25+ Treg expansion. FIG. 2A shows the frequency of colonization with S. aureus at 3 days, 1, 2, 4, and 8 weeks, in the groups of children above and below the median ratio of CD25+ Tregs. We observed that the children who had a CD25+ Treg ratio above the median were significantly more frequently colonized by S. aureus at 3 days and at 4 weeks than the children with a CD25+ Treg ratio below the Median. This was not observed for any other bacterial group or species (data not shown).
[0044] In FIG. 2B it is shown the S. aureus colonization pattern of infants belonging to each of the quartile with respect to CD25+ Treg expansion. A ‘dose-response’ pattern was observed with the 4th quartile showing the most rapid acquisition. In this group, 45% of the children were colonized by S. aureus by day 3 and 91% by 4 weeks. None ...
example 3
S. aureus does not Induce a General Activation of T-Cells
[0046] Apart from being a marker of CD25+ Tregs, CD25 is also expressed by activated T-cells. FIG. 3A shows Ratio (4 months / cord blood) of number of CD4+CD25+CTLA-4− T-cells (cells that recently have been activated, expressing CD25 on the surface, but without the intracellular expression of CTLA-4. These cells are not able to suppress helper T-cell functions). In order to exclude that S. aureus induces activation of T-cells rather than generation of CD25+ Tregs, the colonization frequency in children was compared with a ratio of the numbers of CD4+CD25+CTLA-4− T-cells above and below the median (2.03). We observed no difference in S. aureus colonization between the groups of children at 3 days, 1, 2, 4, and 8 weeks, above and below the median, neither for total S. aureus (FIG. 3B) nor for toxin-producing S. aureus (FIG. 3C). These results support that toxin-producing S. aureus induce an increase in CD25+ Treg counts and not o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
| immune reactivity | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com