Topical Compositions Containing Phosphorylated Polyphenols
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Phosphorylation of Grape Seed Tannin
[0061]100.0 g. of grape seed tannin (TANFOS 167-134)) was dissolved in 300 mL of water at room temperature under nitrogen atmosphere. The solution was brought to a pH of 9 with NaOH 29%, and 26.6 mL phosphorous oxychloride was added to the solution over a period of 1.5 hours. During this addition, more NaOH 29% was added to maintain the pH at 9. After the addition, the reaction mixture was stirred overnight, and analysed by HPLC. The product was then acidified with HCl 25% to a pH of 3.1 and isolated via spray drying to obtain a pink powder.
example 2
Phosphorylation of Resveratrol
[0062]The structure of the compound known as resveratrol (3,4,5-trihydroxystilbene) is as follows:
[0063]Phosphorylation can be achieved, for example, by the procedure disclosed by Pettit et al., J. Med. Chem. 2002, 45, 2534-2542). A solution of resveratrol (25 mmols, 5.7 g) and dimethylaminopyridine (7.5 mmols, 0.93 g) in 100 mL of acetonitrile is cooled under nitrogen up to −10° C. After 10 minutes, CCl4 (375 mmol, 36.2 mL) and DIEA (159 mmols; 27.7 mL) and the mixture maintained under stirring for 30 minutes. Dibenzylphosphite (113 mmols, 25.0 mL) is added and the mixture stirred for an additional 12 hours at room temperature. The course of the reaction is monitored by TLC (Silica F254, eluent ethyl acetate / n-hexane 80 / 20 v / v). One liter 0.5 M KH2PO4 is added, and the mixture then extracted with ethyl acetate. The resulting product, tri(dibenzylphosphate) resveratrol is purified by filtration on a silica gel, washing first with a mixture of ethyl acet...
example 3
Solubility of Resveratrol Triphosphate
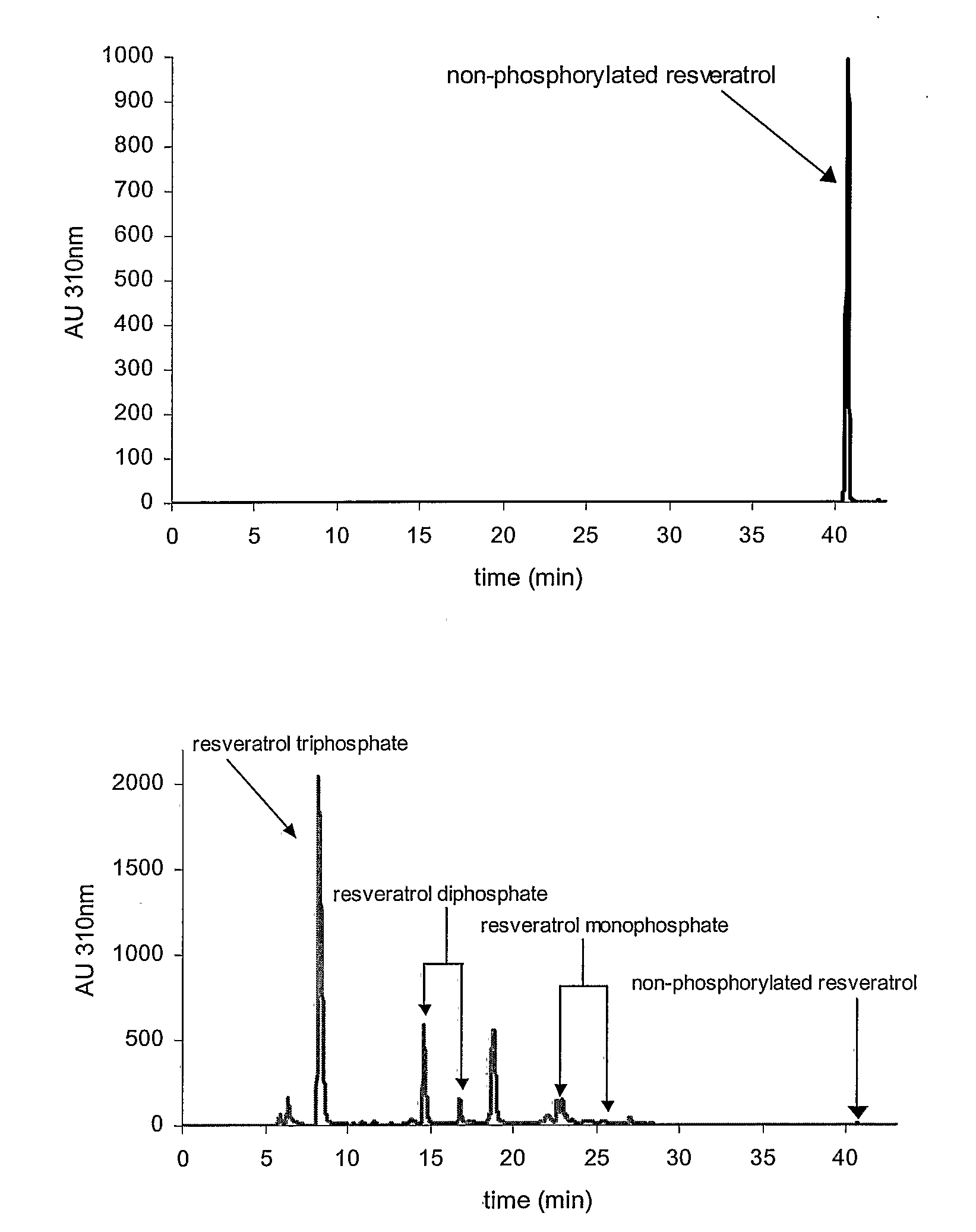
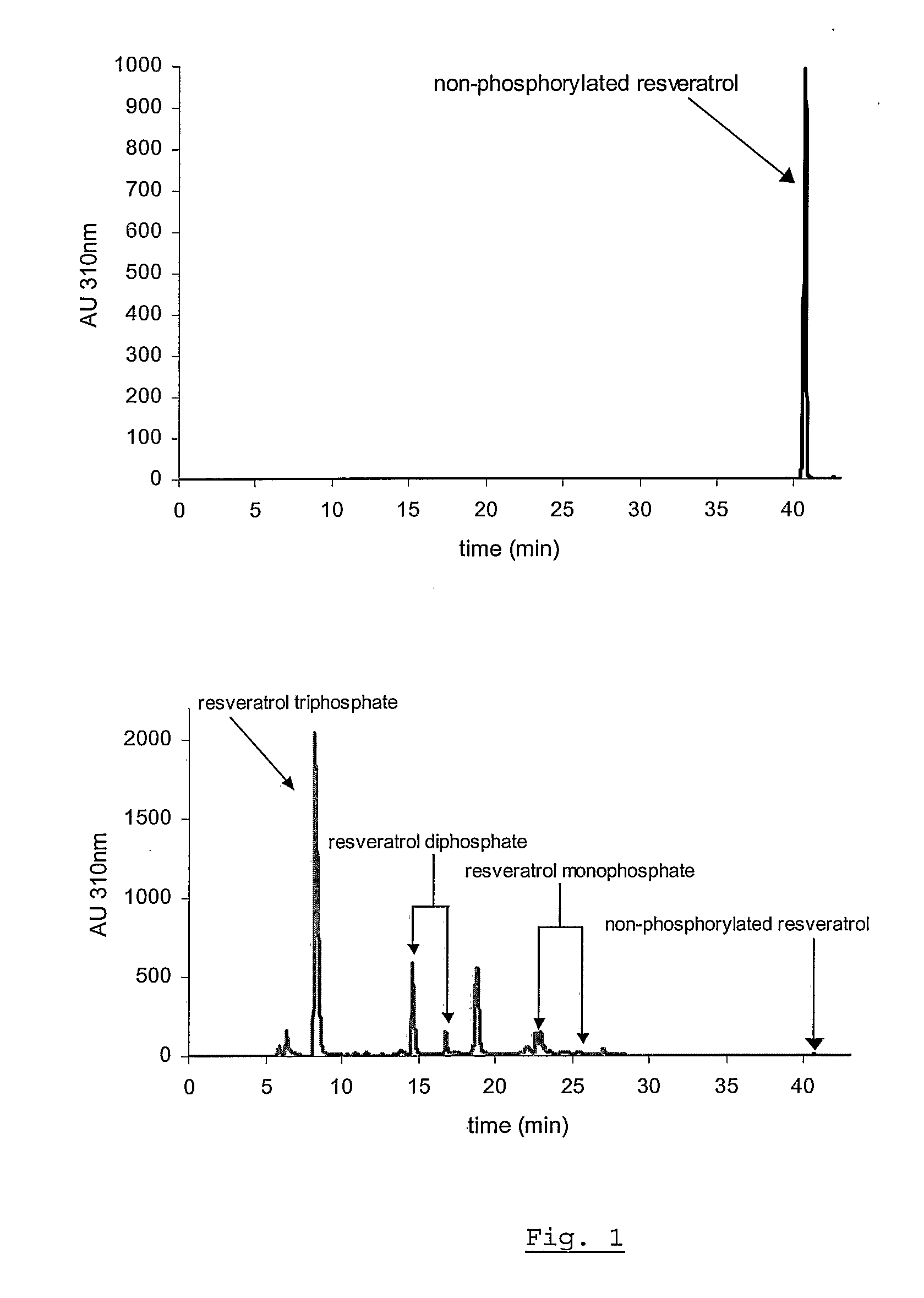
[0066]The water solubility of resveratrol is tested at pH 7 in 1 mM potassium phosphate buffer at room temperature. Ten mg of resveratrol (M.W. 228.24) are suspended in 10 ml of buffer. This suspension, corresponding to a concentration of 1 g / l or 4.38 mM, is agitated for 24 hours in a rotary shaker at 25° C. in the dark. The sample is centrifuged at 14,000 rpm for 5 minutes and an OD of 6.26 is determined at 305 nm wavelength at which the ε mM of resveratrol has a value of 28.1. Assuming complete solubility of resveratrol, the OD that should have been obtained is 123.1 (4.38×28.1). With an observed OD of 6.26, the percentage of dissolved resveratrol is 5.1% (6.26×100 / 123.1) or 5.1 mg / l.
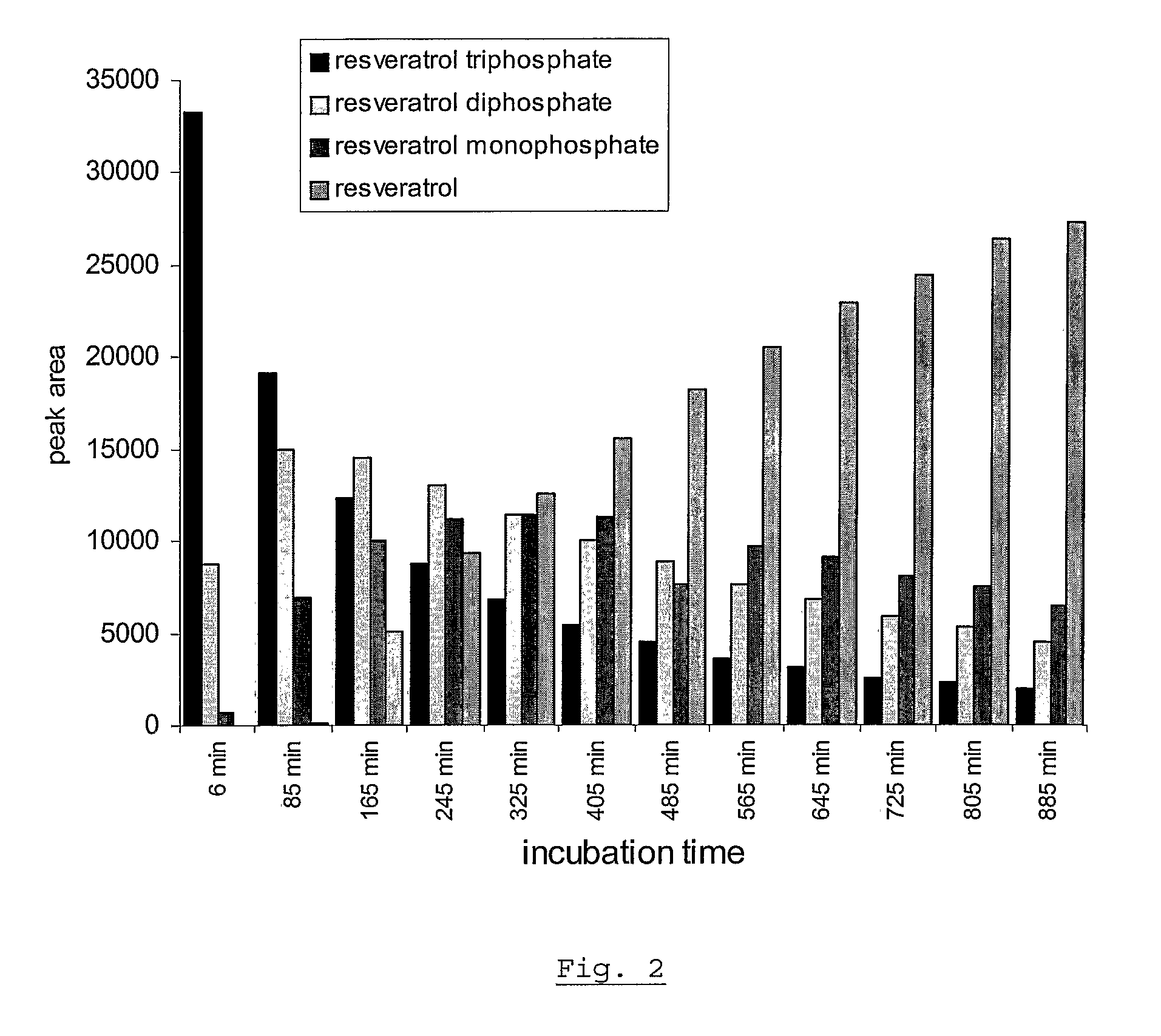
[0067]In contrast, the solubility and stability of the resveratrol triphosphate tested under the same conditions, in water of different pH, in the range of 4 to 9, at room temperature. The solubility of the resveratrol triphosphate is observed to be >30% w / v, sho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility (mass) | aaaaa | aaaaa |
| Hydrolysable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com