Treatment of tumors
a tumor and tumor technology, applied in the field of biotechnology, to achieve the effect of convenient synthesizing and us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Material and Methods
[0075]PEPTIDE SYNTHESIS: The peptides as mentioned herein such as LQG, AQG, LQGV (SEQ ID NO:1), AQGV (SEQ ID NO:2), LQGA (SEQ ID NO:37), VLPALP (SEQ ID NO:4), ALPALP (SEQ ID NO:51), VAPALP (SEQ ID NO:52), ALPALPQ (SEQ ID NO:53), VLPAAPQ (SEQ ID NO:54), VLPALAQ (SEQ ID NO:55), LAGV (SEQ ID NO:3), VLAALP (SEQ ID NO:59), VLPALA (SEQ ID NO:56), VLPALPQ (SEQ ID NO:43), VLAALPQ (SEQ ID NO:62), VLPALPA (SEQ ID NO:57), GVLPALP (SEQ ID NO:58), VVCNYRDVRFESIRLPGCPRGVNPVVSYAVALSCQCAL (SEQ ID NO:63), RPRCRPINATLAVEKEGCPVCITVNTTICAGYCPT (SEQ ID NO:64), SKAPPPSLPSPSRLPGPS (SEQ ID NO:65), LQGVLPALPQVVC (SEQ ID NO:66), SIRLPGCPRGVNPVVS (SEQ ID NO:67), LPGCPRGVNPVVS (SEQ ID NO:68), LPGC (SEQ ID NO:61), MTRV (SEQ ID NO:6), MTR, and VVC are prepared by solid-phase synthesis (R. B. Merrifield, J. Am. Chem. Soc., 85:2149-2165 (1963)) using the fluorenylmethoxycarbonyl (Fmoc) / tert-butyl-based methodology (Atherton, 1985) with 2-chlorotrityl chloride resin (Barlos et al., Int. J. Pepti...
example ii
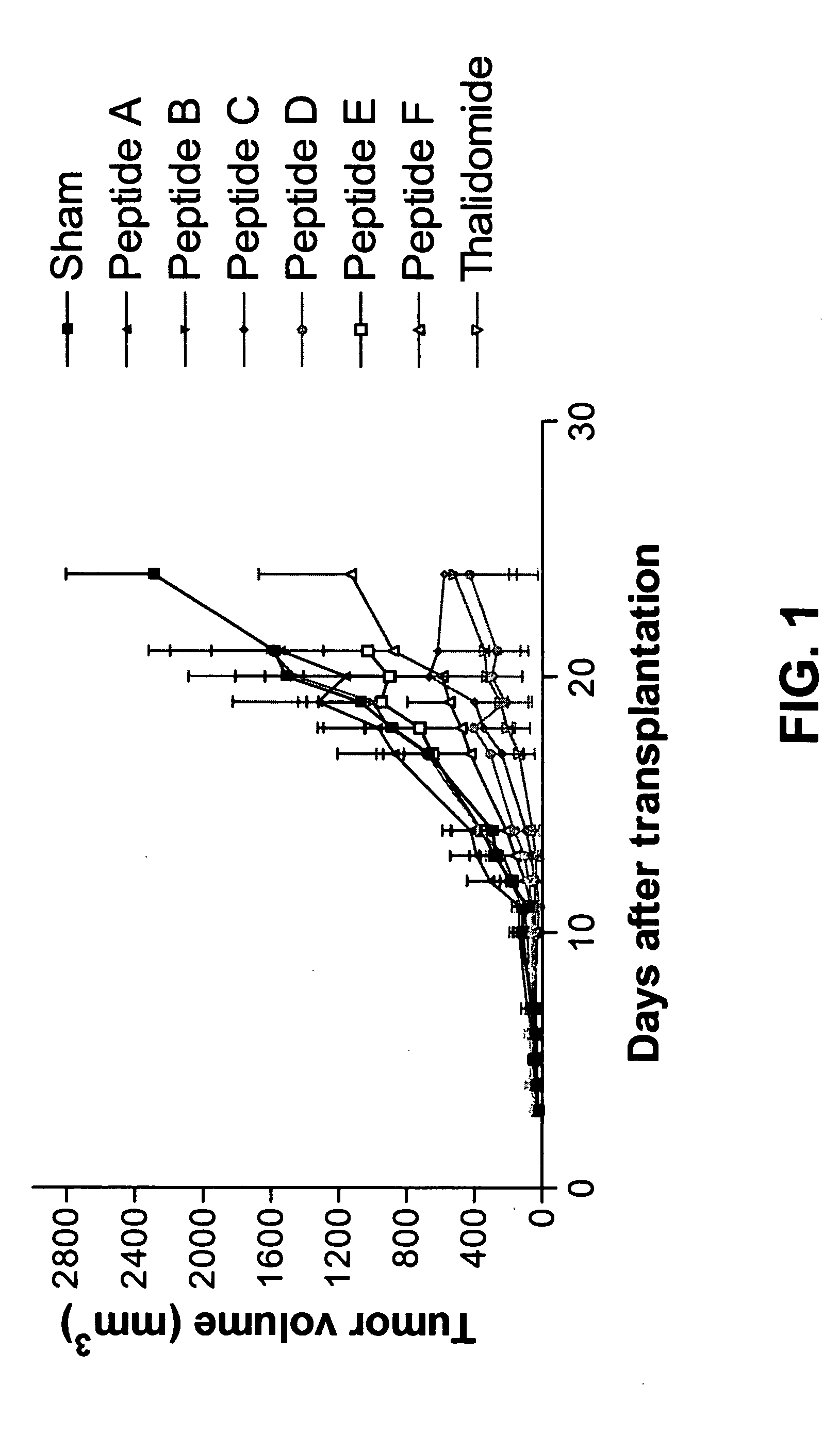
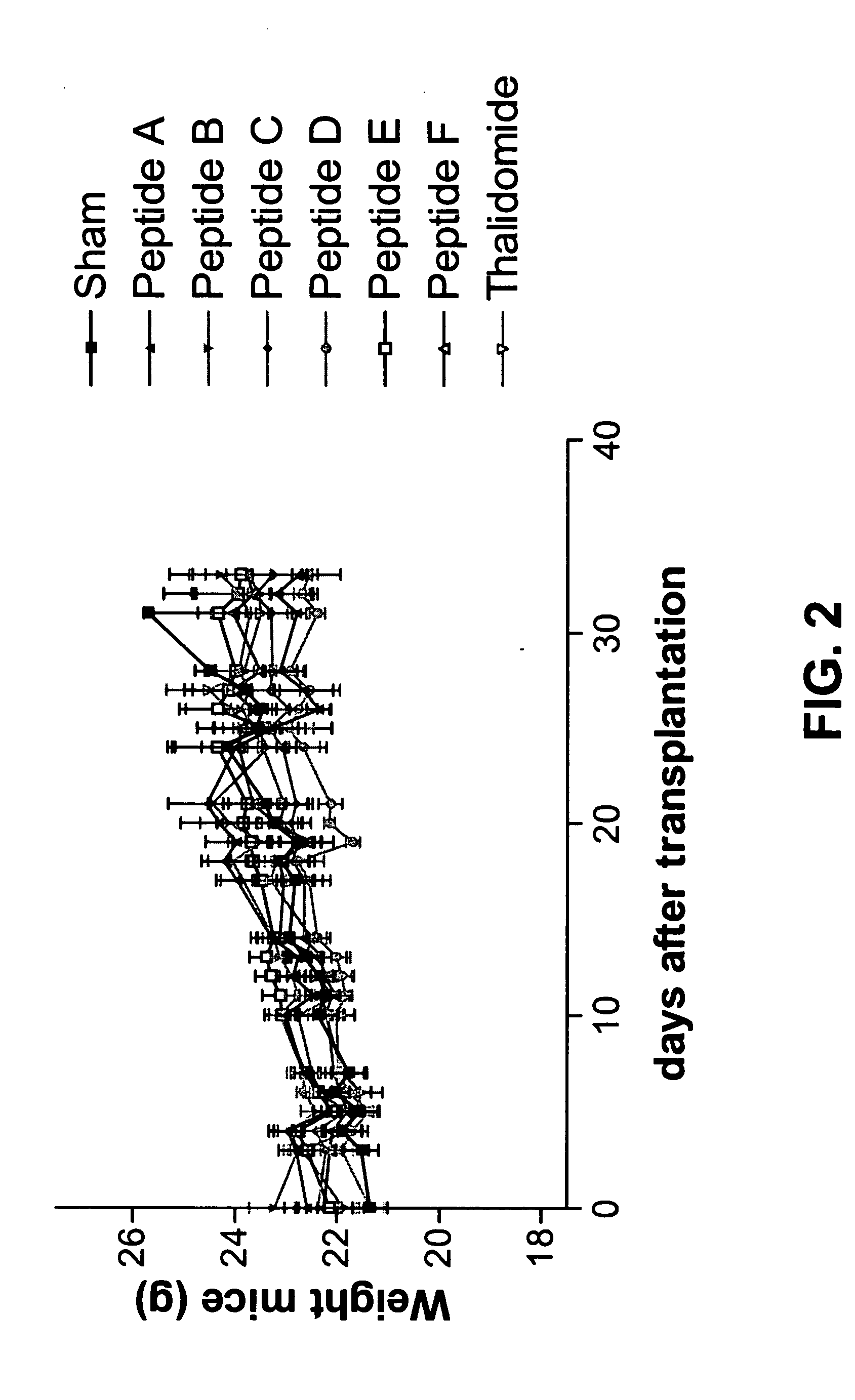
[0078]Animals and tumor model: Mice (C57B16) are transplanted with the syngeneic Lewis Lung Carcinoma (LLC) subcutaneously.
[0079]Treatment modalities: mice are treated with small peptides or control substances beginning on the day of transplantation by i.p. injection. Mice are injected on Monday, Wednesday and Friday, until end of experiment. Experiment ends are based on: tumor size >20 mm, suffering of the animal, weight loss over 20%, ulcerations etc. Otherwise the experiment is terminated eight weeks after start.
Method of Efficacy Evaluation
Assessment of Tumor Response
[0080]Tumor diameter is measured in two directions by caliper measurements and tumor volume (V) is calculated using the formula V=0.4(A2XB) (where B represents the largest diameter and A the diameter perpendicular to B). The classification of tumor response is: progressive disease (PD), increase of tumor volume (>50%) within seven days (between day 3 and day 10 after begin of treatment); stable disease, tumor volume...
example iii
Method of Testing
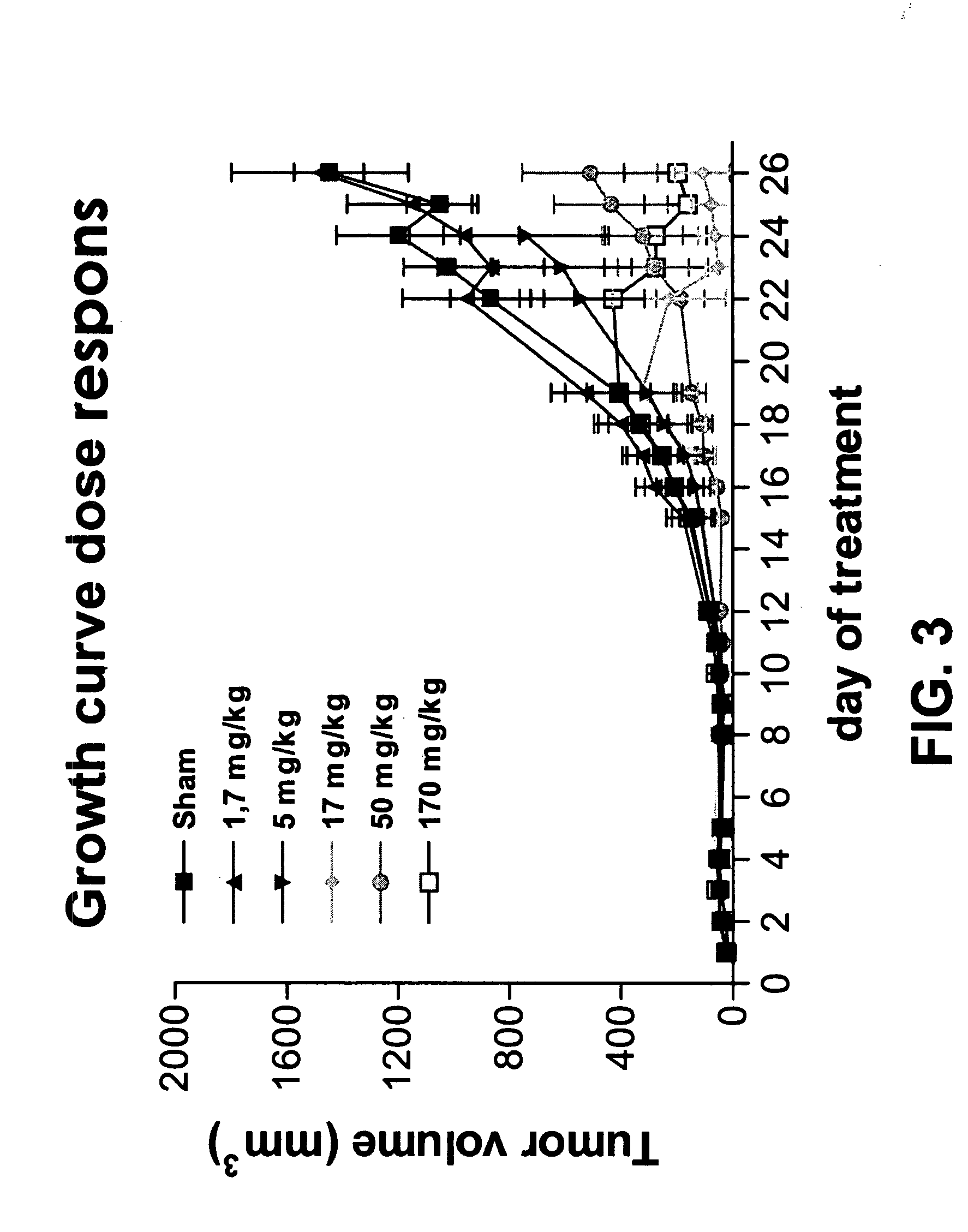
[0092]Animals and tumor model. Mice (C57B16) are transplanted with the syngeneic Lewis Lung Carcinoma (LLC) subcutaneously. Treatment is stared 24 hours after implantation. The number of animals is eight per group to increase the power of this study. As we are starting the treatment when no tumor is palpable the change of error is larger compared to a study with established tumors. Treatment modalities: mice are treated with small peptides or sham control beginning one day after transplantation by i.p. injection. Mice will be injected on Monday, Wednesday and Friday, until end of experiment. Experiment ends based on criteria as tumor size >20 mm, suffering of the animal, weight loss over 20%, ulcerations. Otherwise the experiment will be terminated eight weeks after start.
Method of Efficacy Evaluation
Assessment of Tumor Response
[0093]Tumor diameter is measured in two directions by caliper measurements and tumor volume (V) is calculated using the formula V=0.4(A2XB) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com