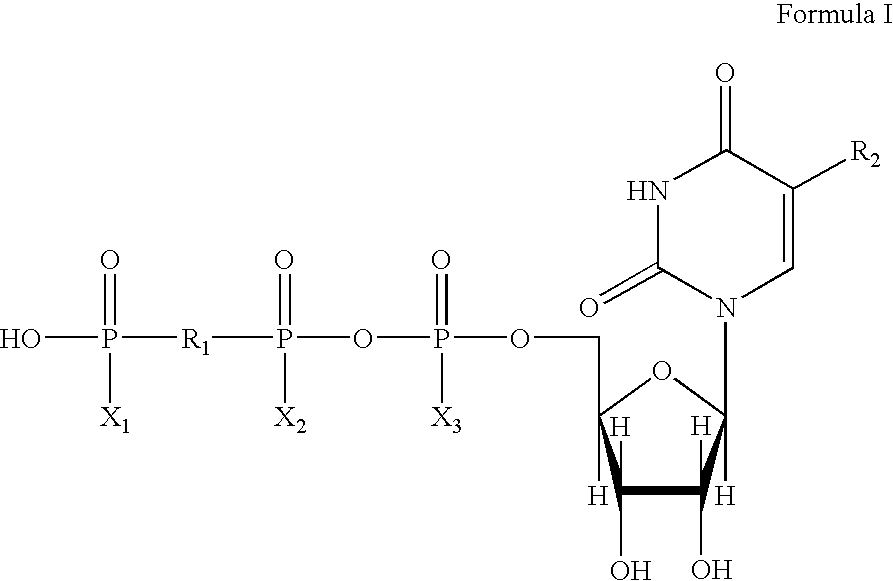
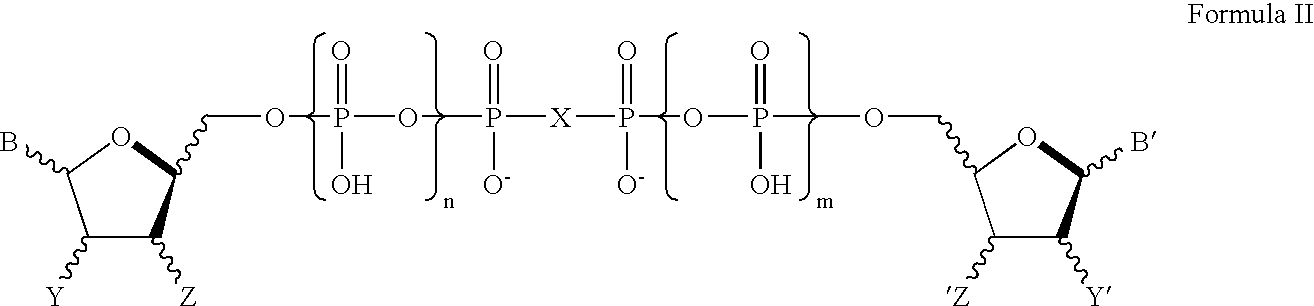
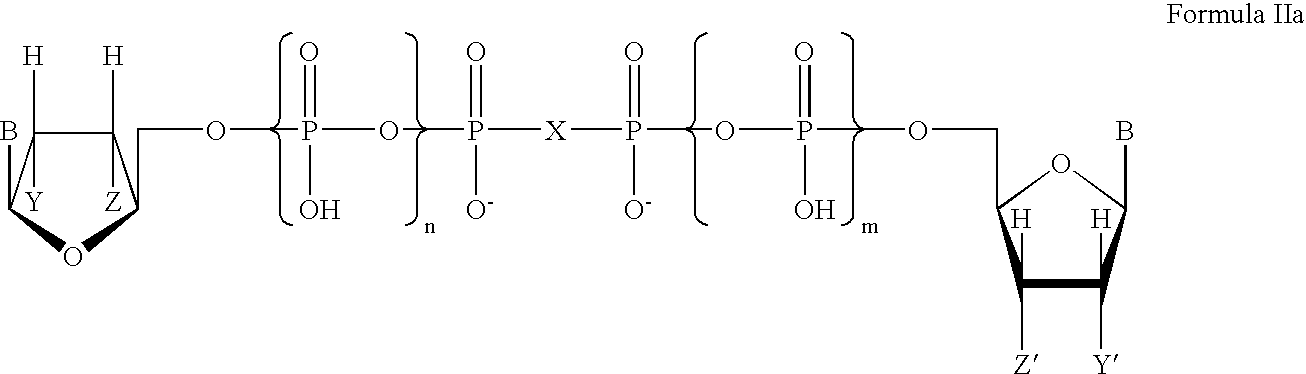
Method of promoting cervical and vaginal secretions
a vaginal secretion and vaginal secretion technology, applied in the direction of biocide, drug composition, sexual disorder, etc., can solve the problems of endometrial hyperplasia and carcinoma, inability to cure surgery, unwanted risks and side effects, etc., to stimulate vaginal and cervical secretions, and increase vaginal and vaginal secretions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vitro Short Circuit (Isc) Measurements
[0150]The compound UTP is a potent agonist of P2Y2 and / or P2Y4 purinergic receptors in cervical and vaginal tissue preparations by evaluation in vitro by administering UTP to the tissue culture sufficient to achieve concentrations of UTP on the mucosa of from about 10−7 to about 10−1 moles / liter. (Rojanasakul, Y., et al., Pharm. Res. 9:1029-34 (1992); Bechgaard, E., et al., Int. J. Pharm. 106:237-242 (1994); Gipson, I., et al., Biol. Reprod. 56:999-1011, (1997)). Specifically, ovariectomized female white albino New Zealand rabbits are sacrificed and vaginal tissue is removed. The tissue is mounted on a supporting ring and clamped in an Ussing chamber. Isc is measured as flowing from the epithelial side to the serosal side of the tissue. Approximately half of this current corresponds to chloride movement through the membrane and hence, this is an accurate measure of the corresponding fluid movement.
example 2
In Vivo Study in Rabbits
[0151]The compounds of the invention are evaluated in vivo by administrating UTP, or any of the other P2Y2 and / or P2Y4 agonists of the present invention to an animal in an amount sufficient to achieve concentrations of P2Y2 and / or P2Y4 agonist on the cervical and / or vaginal mucosa of the animal of from about 10−7 to about 10−1 moles / liter (Richardson, J., et al., Int. J. Pharm. 56:29-35 (1989)). Specifically, ovariectomized female white albino New Zealand rabbits are dosed with a P2Y2 and / or P2Y4 agonist such as any of the compounds of the present invention. A vaginal smear is then obtained with a cotton swab. The sample is appropriately prepared, an ELISA or a colorimetric dot blot method is run on the sample, and the relative amounts of representative cervical mucins are determined as compared to non-ovariectomized controls. (Gipson, I. et al., Biol. Reprod. 56:999-1011 (1997)).
example 3
In Vivo Study Using Ovarectomized Cynologous Monkeys
[0152]The compounds of the present invention are evaluated in vivo with an animal model of vaginal dryness as follows. Ovariectomized cynomolgus monkeys are examined before treatment and graded subjectively using a fabinal atrophy index. The animals are then dosed intravaginally with 100 to 300 μl mist containing 10−2 to 1 moles / liter P2Y2 and / or P2Y4 agonist, such as any of the compounds of the present invention. After 10, 20, 30, 60 and 90 minutes the animals are subjected to a gynecological exam and graded by qualified medical professions with the vaginal atrophy index on a scale of 1 to 5, including a measurement of fluid pH. (Hubbard, G. et al., Lab Animal Sci. 47, 36-39, (1997)).
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com