Method and System for Verification of Source Data in Pharmaceutical Studies and Other Applications
a source data and system technology, applied in the field of method and system for source data verification in pharmaceutical studies and other applications, to achieve the effect of improving the speed and accuracy of the process of checking, reducing the interval, and improving the efficiency of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
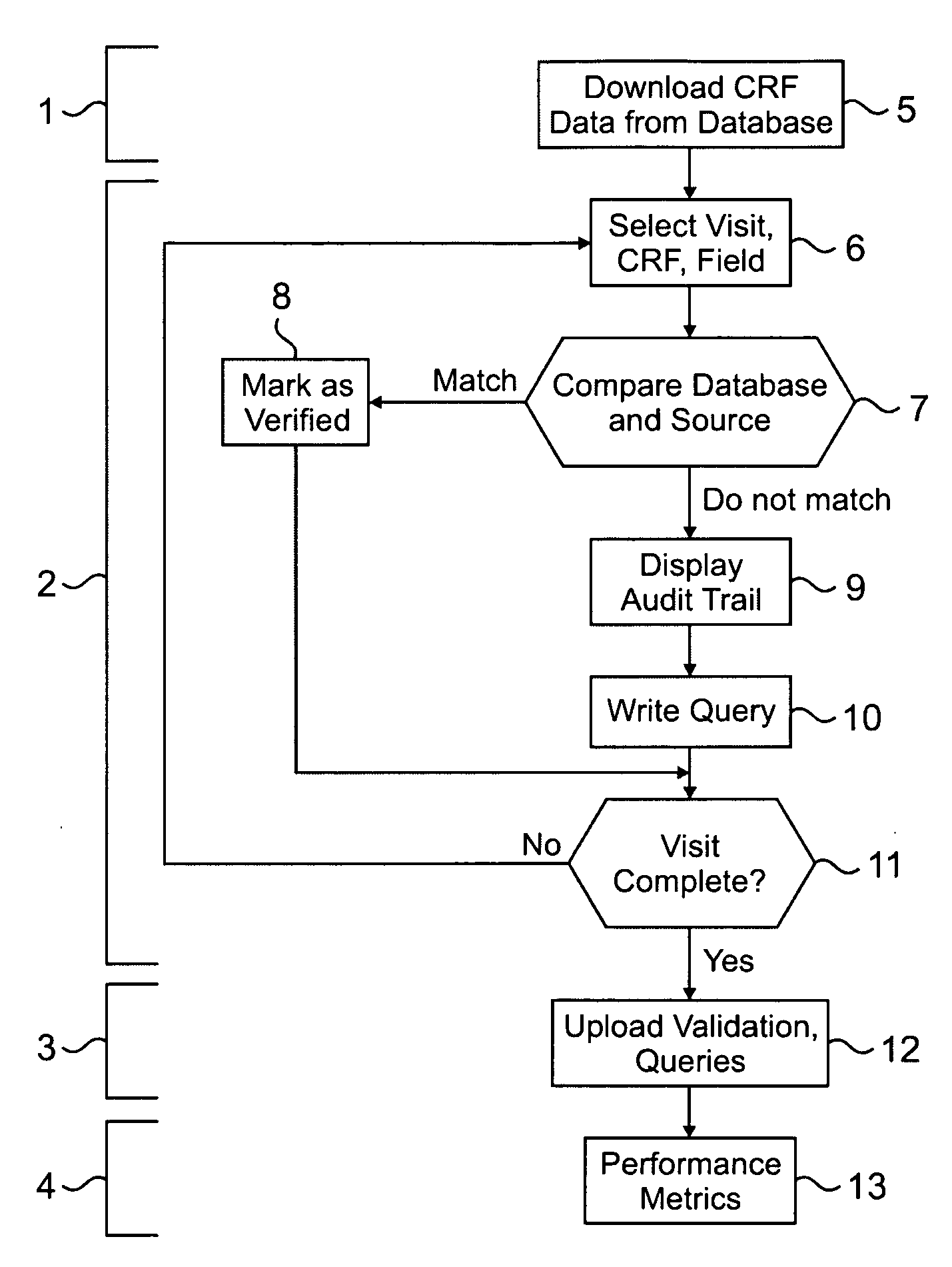
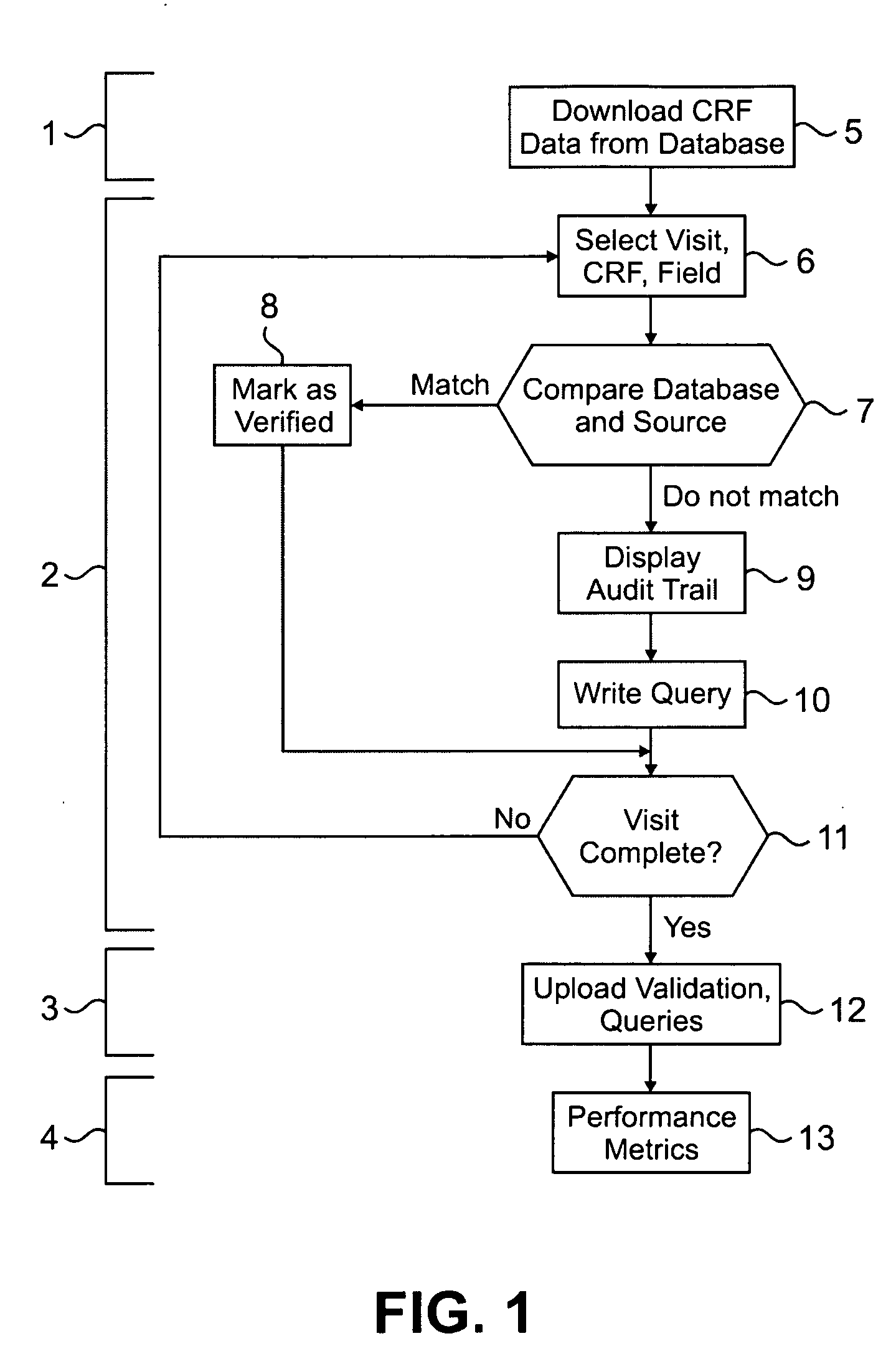
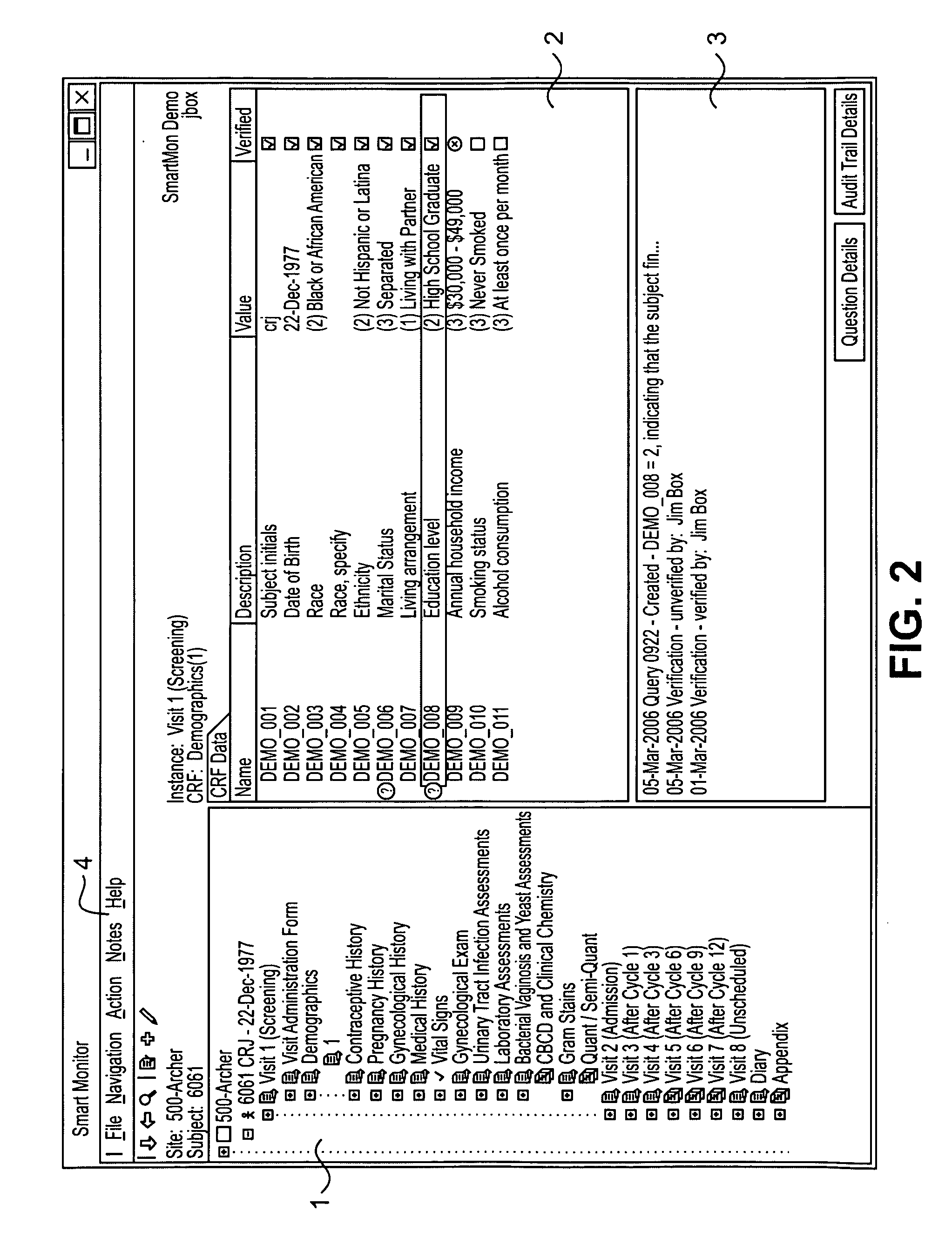
[0036]Clinical trials are generally highly complex processes that involve collection of many thousands of data elements from multiple clinical sites, laboratory facilities, regulatory agencies, and often outside vendors such as companies supplying test drugs. Many of the foregoing may be in different countries, which present the challenges of different cultures, languages, time zones, and other differences that complicate the ability to effectively manage such diverse participants in a clinical evaluation. The quality of the data collected in such circumstances is of paramount importance, because accurate data are necessary to demonstrate the efficacy and safety of any pharmaceutical product being evaluated. Failure to optimize data quality slows study progress; requires a greater number of patients in order to demonstrate an effect; impairs the ability of a manager to change a study based on what already has occurred in the course of the study; and slows regulatory filings because ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com