Compunds and compositions that cause non-apoptotic cell death and uses thereof
a technology of compositions and compounds, applied in the field of gene-selective antitumor drugs, can solve the problems of difficult to target effectively with small molecules, difficult to realize the approach, and inability to inhibit oncogenic proteins, etc., and achieve the effects of reducing the level of vdac protein, causing significant resistance to erastin, and reducing the effect of vdac protein levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Engineered Human Tumor Cells
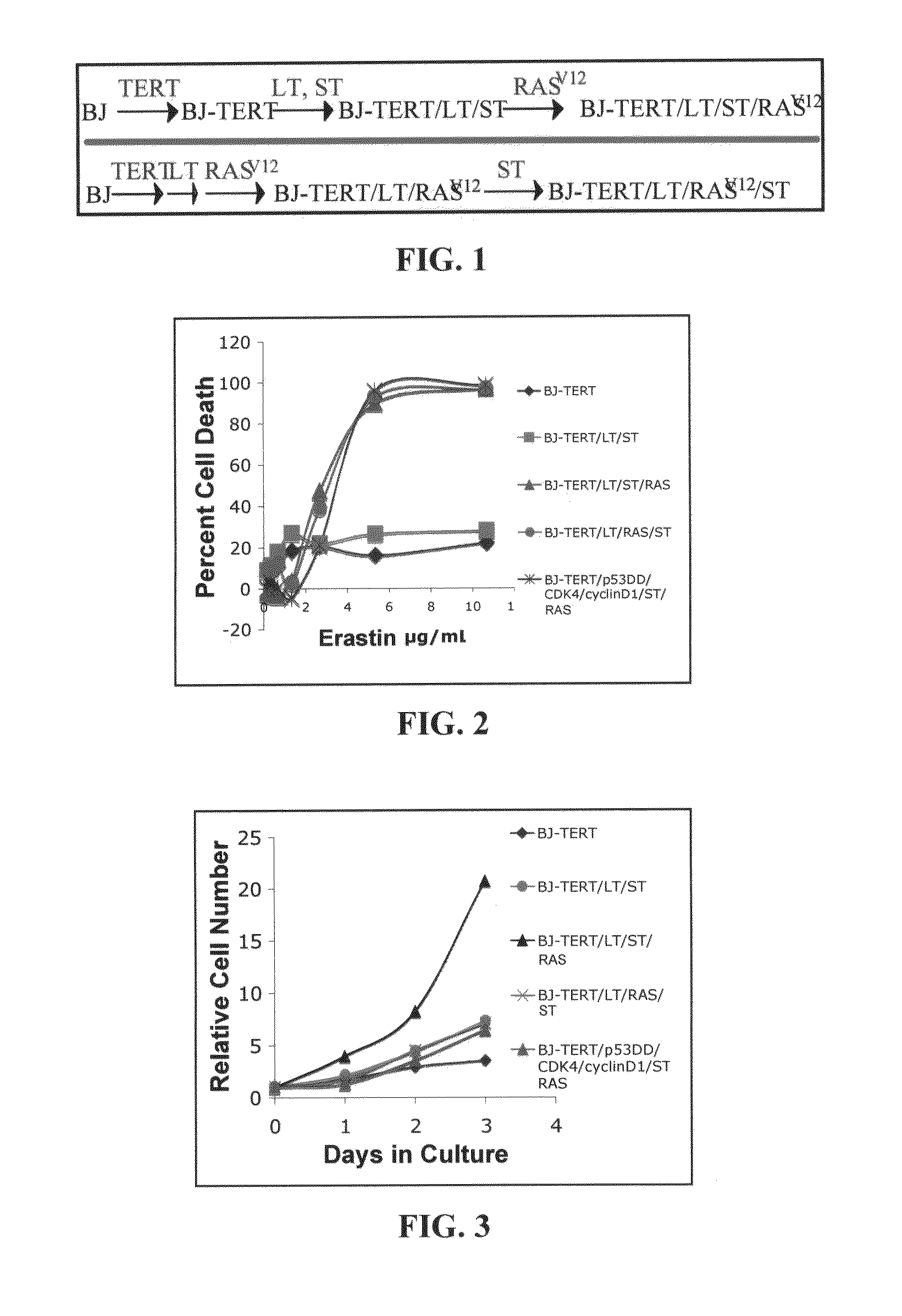
[0293]Primary human cells can be converted into tumorigenic cells by introduction of vectors expressing hTERT, oncogenic RAS, and other proteins that disrupt the function of p53, RB, and PP2A55-60. Such engineered human tumorigenic cells and their precursors (FIG. 1), were created from primary human foreskin fibroblasts. Characteristics of these cells reported previously include doubling time, resistance to senescence and crisis in culture, response to irradiation, ability to grow in an anchorage-independent fashion, and ability to form tumors in mice56, 57, 60. These cells were used to discover RAS-selective lethal compounds, including a compound named erastin.
Cell Culture and Western Blotting:
[0294]BJ-TERT / LT / ST / RASV12 cells were cultured as described (Dolma, S., Lessnick, S. L., Hahn, W. C. & Stockwell, B. R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 3: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Cell death | aaaaa | aaaaa |
| Hydrophilicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com