Combination therapy using active immunotherapy
a technology of immunotherapy and combination therapy, applied in the direction of antibody medical ingredients, drug compositions, vertebrate antigen ingredients, etc., can solve the problems of severe immune response effect, not killing all cancer cells in the patient, and compromising the quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Quantification of Sorafenib and Sunitinib in Biological Fluids
[0111]Sunitinib malate and sorafenib tosylate were supplied by euroasia chemicals PVT. LTD., Mumbai (India).
1.1. Sample Preparation for Sunitinib Quantification
[0112]To quantify sunitinib in blood serum or in cell culture medium, 10 μl of 50% acetonitril (Acros, Geel, Belgium) was added to 50 μl serum or medium in brown glass tubes to protect the photo-unstable sunitinib from light, and mixed for 10 seconds. The proteins were precipitated with 40 μl 100% acetonitril (Acros, Geel, Belgium), centrifuged and filtered through a 0.2 μm PVDF filter. 10 μl was directly injected to the HPLC system.
1.2. HPLC-Conditions:
[0113]The HPLC system consisted of a Binary HPLC pump (Shimadzu LC10aVP), a Shimadzu SIL-10aVP autosampler, a Shimadzu CTO-10asVP column oven and a Shimadzu SPD10aVP detector. Data acquisition and analysis was performed using the Shimadzu Class-VP 7.3 software. Chromatographic separation was carried out on a reverse...
example 2
Alteration of Expression of Vaccination Relevant Genes During TKI Treatment
2.1. Alteration of Gene Expression Profiles of Human Tumor Cell Lines In Vitro
[0118]Genome-wide mRNA expression was measured by Affymetrix microarrays. The human renal cell carcinoma cell line A498 was cultured in the presence of sorafenib and sunitinib. Gene expression for a selection of tumor associated antigens and genes involved in antigen presentation to T lymphocytes was compared with untreated cells to determine whether these tyrosine kinase inhibitors (TKIs) might have the potential to cause altered presentation of antigens in vitro.
[0119]The human renal cell carcinoma cell lines A498 and RCC068 were cultured in RPMI medium (5% FCS (Biochrom, Berlin, Germany), 5% HS (PromoCell, Heidelberg, Germany)). Human serum was added as a supply of ligands influencing signaling pathways, which might be altered by TKIs. 40 h after seeding, the experiment was started by addition of TKIs to the culture flasks. The f...
example 3
In Vitro Alteration of T-Cell Activation by Kinase Inhibitors
3.1. Mouse T-Cell Activation
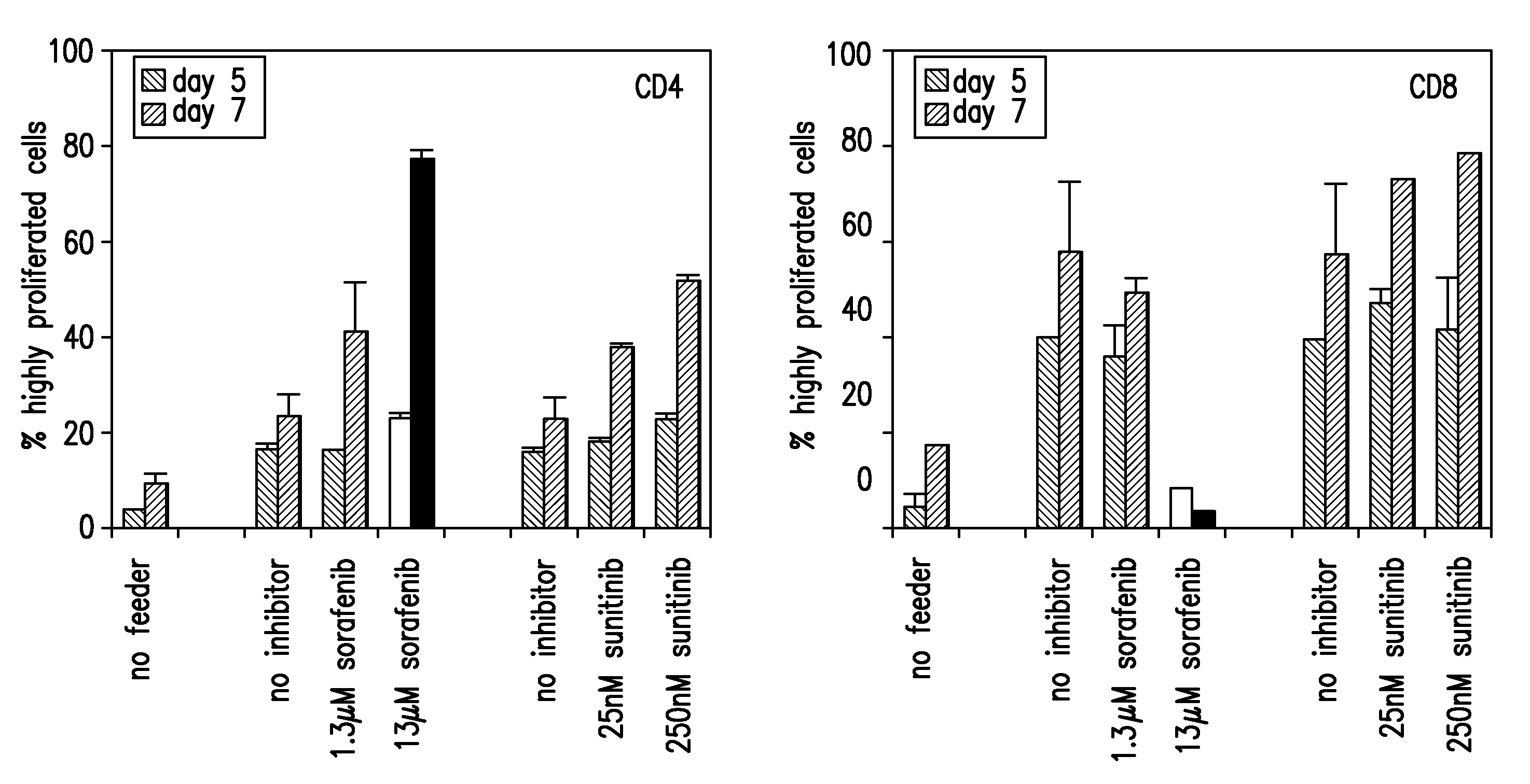
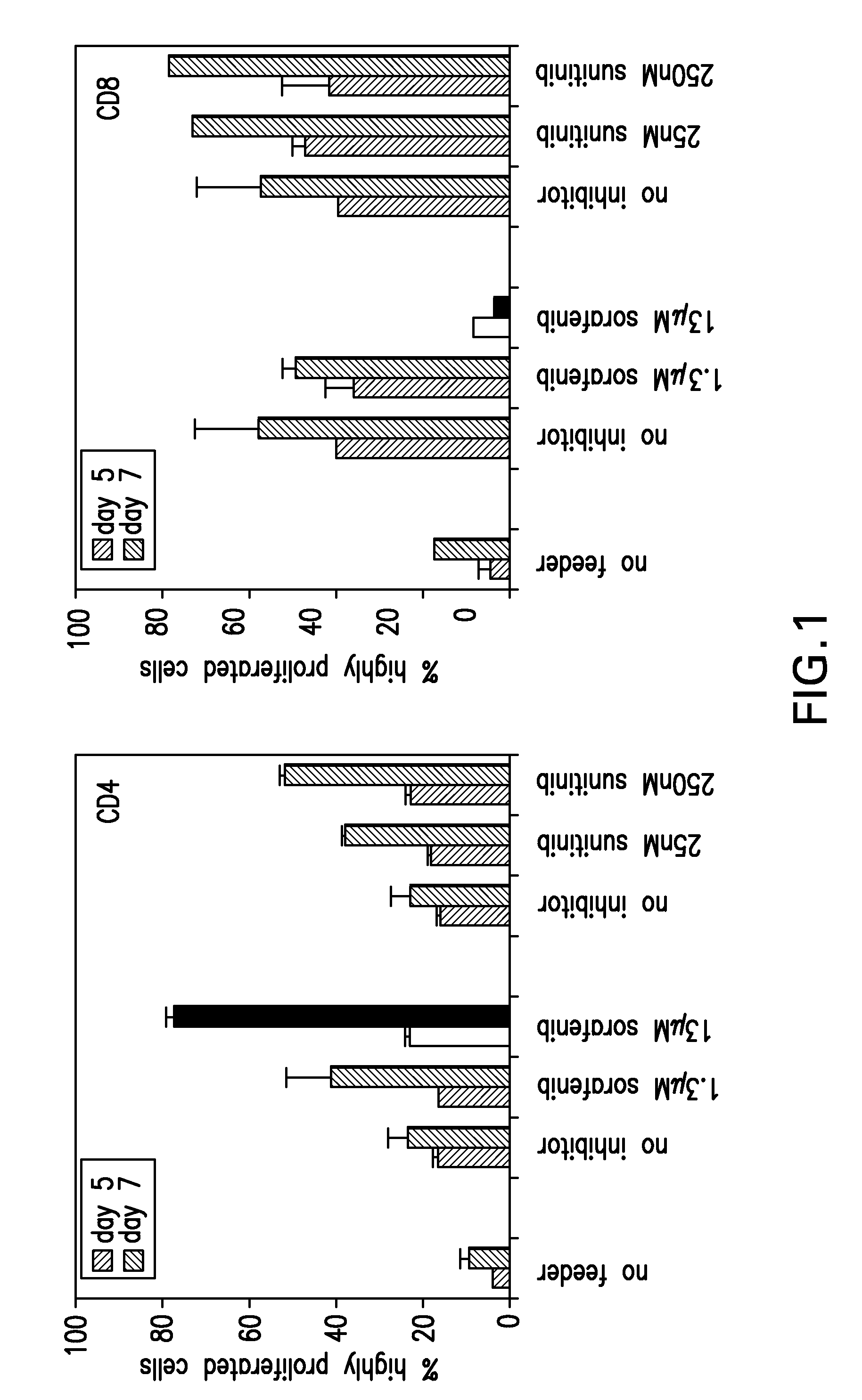
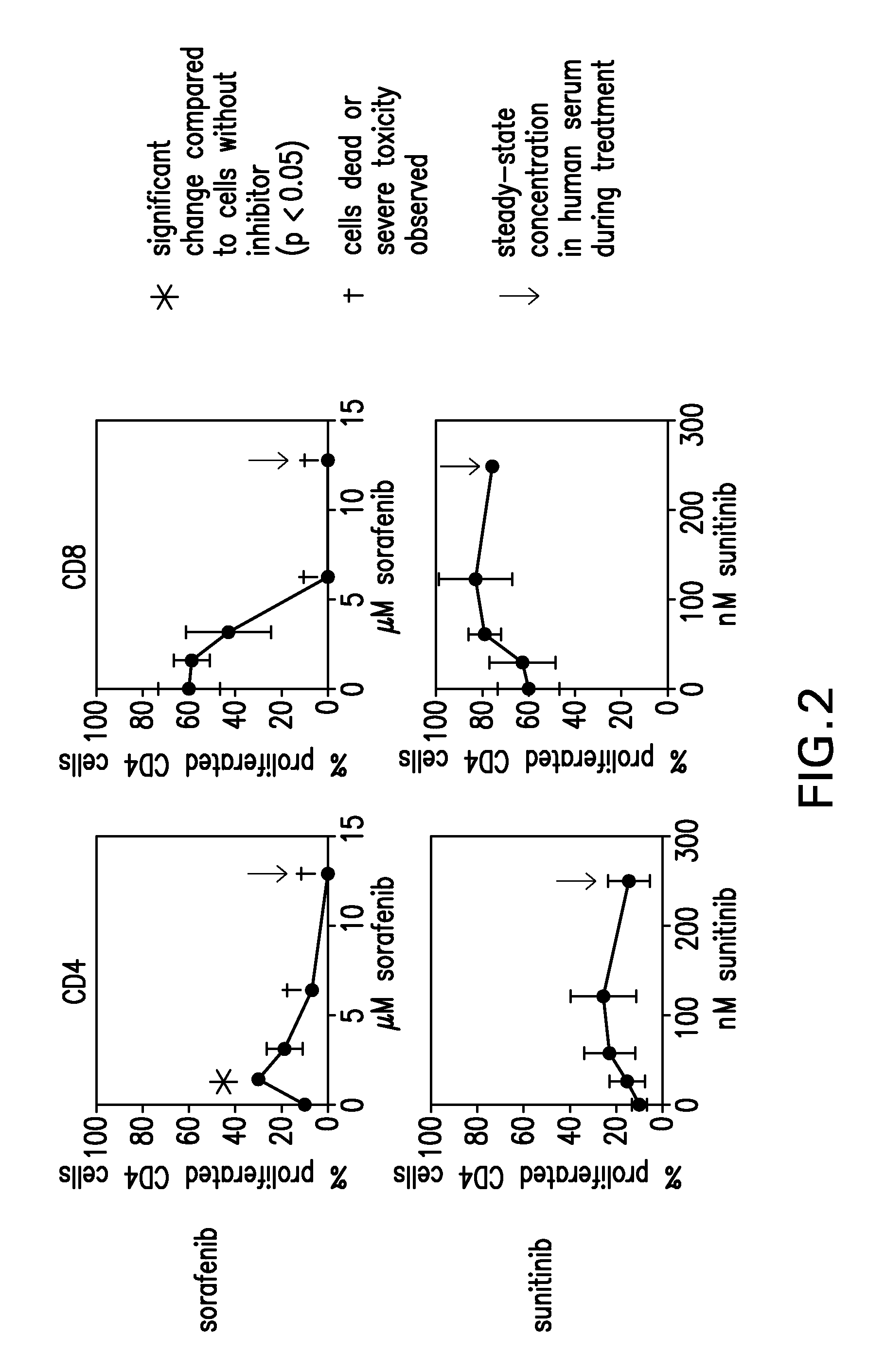
[0135]To test whether the presence of sorafenib or sunitinib has an influence on mouse T-cell responses, alloreactive T-cells responses (CD4 and CD8) were assessed in mixed lymphocyte reactions (MLR).
[0136]Allogenic responses are the most potent and strong immune responses and they are easy to generate in the mouse system due to the availability of congenic mouse strains differing in their H2 alleles. Therefore, first hints on the influence of sunitinib and sorafenib on immune responses can be drawn from in vitro mixed lymphocyte cultures.
3.1.1. Mixed Lymphocyte Reaction Assay
[0137]CFSE-labeled spleen cells from C57BL / 6 (H2-b) mice were co-cultured with irradiated splenocytes from BALB / c (H2-d) mice, resulting in the strong allogenic response and proliferation of the C57BL / 6 T cells against H2-d MHC molecules. The proliferation of the T cells results in a diminished CFSE staining of the divided ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com