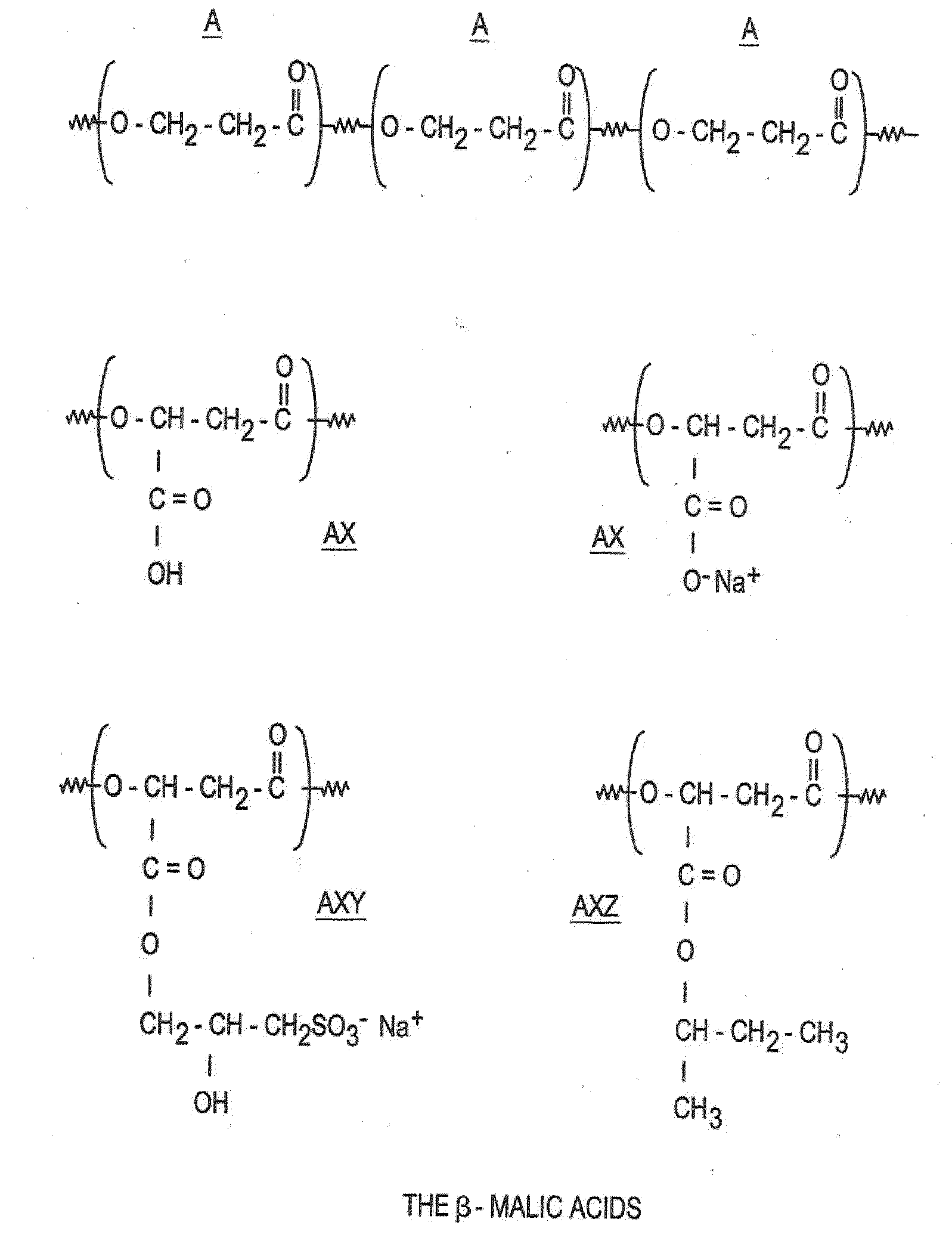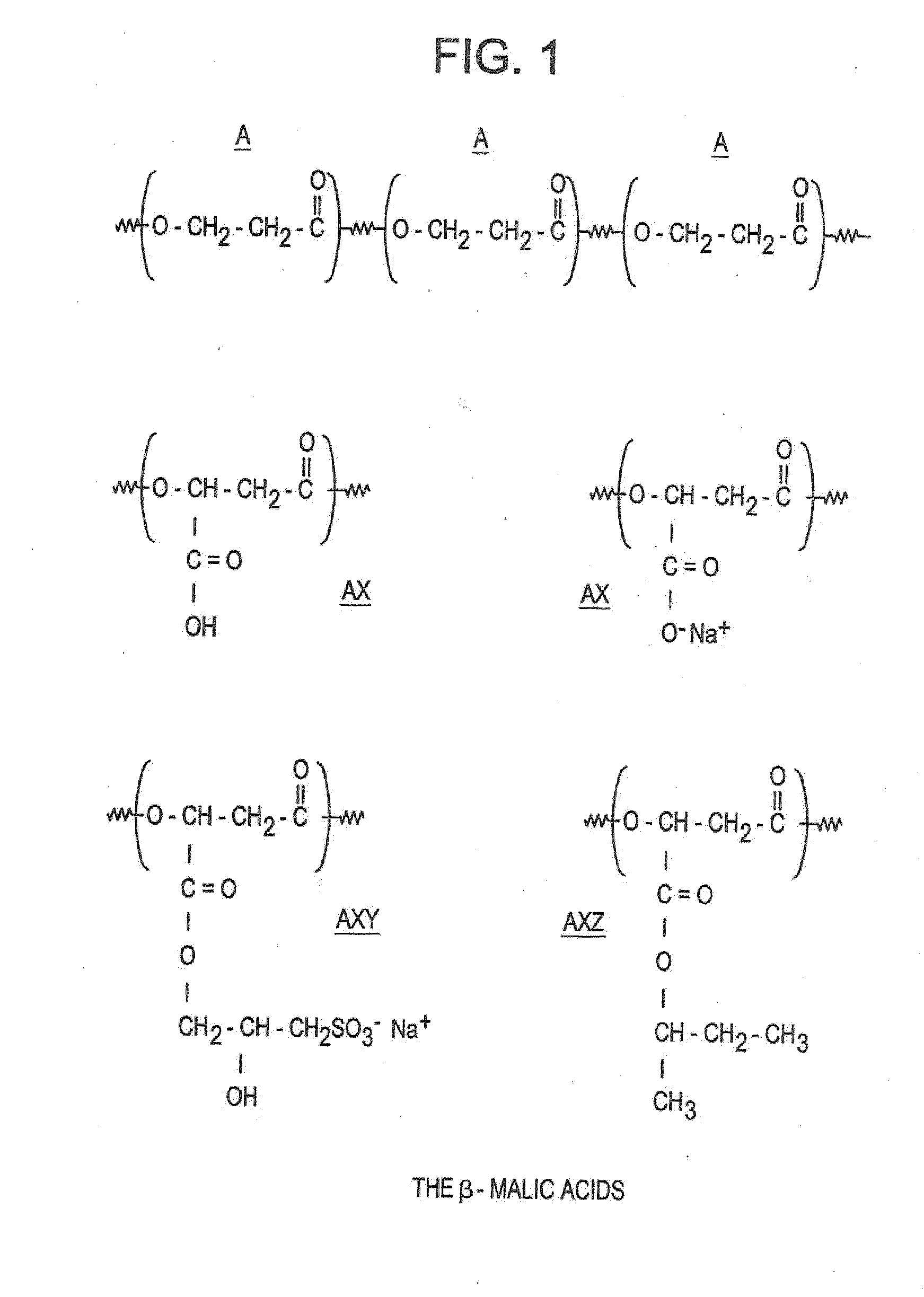Biocompatible polymers, process for their preparation and compositions containing them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Poly(β-Malic Acid) Derivatives from a Non-Polysaccharide Skeleton
[0129]In this example, the polymer of the invention is a copolymer of β-malic acids of general formula (II), the components A of which, substituted by X and / or Y and / or Z, are represented in FIG. 1. In FIG. 1:
[0130]A is —(O—CH2—CH2—CO)—
[0131]X is —COOH or —COO−Na+
[0132]Y is —CO—CH2—CHOH—CH2—SO3H or —CO—CH2—CHOH—CH2—SO3−Na+
[0133]Z is —CO—OCH3—CH(CH2—CH3)—CH3
[0134]x, y and z correspond to: the percentages of the X, Y and Z groups shown in Table I below in relation to the different polymers synthesized.
TABLE IType ofCarboxylicSulfonateHydrophobicReferencepolymergroups = Xgroups = Ygroups = ZRGTA 2010Pcoo−100%0%0%RGTA 2011P1S60%10%10%RGTA 2012P2S75%11%12%
[0135]Pcoo− corresponds to a polymer composed exclusively of carboxylic or carboxylate groups X. P1S and P2S correspond to polymers composed of sulfonate groups Y and hydrophobic butyl groups Z in addition to the groups X.
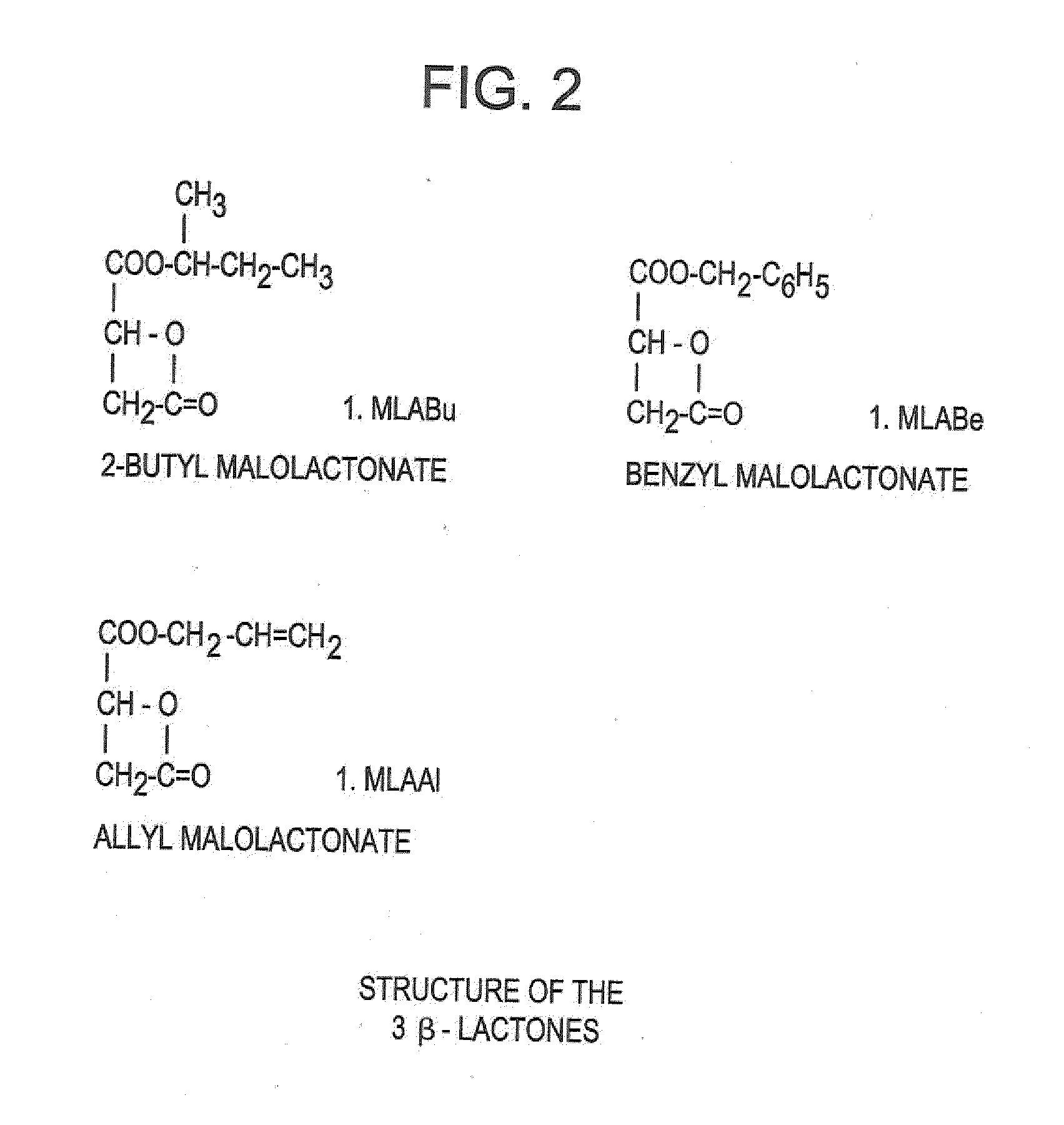
[0136]The synthesis of this polymer p...
example 2
Synthesis of Polysaccharide Polymers Constituted by Substituted Glucose Motif
I—Synthesis of Carboxymethyl Dextran Sulfates Designated CMDS
[0175]In this example, the polymer of the invention is constituted of substituted dextran in which the glucose A motifs substituted by X and / or Y and in which Z is nothing are represented in FIG. 5. The different types of grafting are shown in FIG. 5 in which:
[0176]A is a glucose monomer on which X, Y and Z are grafted by the intermediary of the hydroxyl functions in position 2 and / or position 3 and / or position 4 and / or by the intermediary of the Y groups for Z,
[0177]X is —CH2COOH or —CH2COO−Na+
[0178]Y is —SO3H or SO3−Na+
[0179]Z is a variable group of which several examples are presented below.
[0180]The polymers of type CMDS in which Z=nothing contain multiple types of monomers. The first types of substituted monomers are the carboxymethyl glucose of type A-X substituted in position 2 and / or 3 and / or 4 (motifs presented in FIG. 5). The addition of...
example 3
Measurement of the Anticoagulant Activities of the Polymers of Examples 1 and 2
[0276]The coagulation tests were performed using the Activated Cephalin Time technique or A.C.T. (Biggs, 1972, In: Human Blood Coagulation, Oxford Blackwell Scientific Publications). One hundred microliters of a polymer solution at different concentrations in Owen Koller buffer are incubated for 5 minutes at 37° C. with 100 microliters of plasma poor in platelets and 100 microliters of a solution of rabbit brain cephalin. 100 microliters of 0.25 M calcium chloride are added and the time until appearance of the coagulum is referenced by chronometry.
[0277]As shown in FIG. 11, the polymers of Examples 1 and 2 do not present anticoagulant activities greater than 50 IU / milligram, especially with respect to heparin which was used as a; positive control. It should be noted that all of the values for the anticoagulant activities of the products presented here as examples are lower than 10 IU / milligram of product....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com