Methods and compositions for the specific inhibition of gene expression by double-stranded RNA
a technology of double-stranded rna and composition, which is applied in the direction of immunological disorders, metabolism disorders, extracellular fluid disorders, etc., can solve the problems of complex set of rules, sirna molecules are capable of targeting, early attempts to suppress gene expression using long dsrnas in mammalian systems failed, etc., to achieve the best stability, improve stability, and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Double-Stranded RNA Oligonucleotides
[0110]Oligonucleotide Synthesis and Purification
[0111]RNA oligonucleotides were synthesized using solid phase phosphoramidite chemistry, deprotected and desalted on NAP-5 columns (Amersham Pharmacia Biotech, Piscataway, N.J.) using standard techniques (Damha and Olgivie, 1993; Wincott et al., 1995). The oligomers were purified using ion-exchange high performance liquid chromatography (IE-HPLC) on an Amersham Source 15Q column (1.0 cm×25 cm) (Amersham Pharmacia Biotech, Piscataway, N.J.) using a 15 min step-linear gradient. The gradient varied from 90:10 Buffers A:B to 52:48 Buffers A:B, where Buffer A was 100 mM Tris pH 8.5 and Buffer B was 100 mM Tris pH 8.5, 1 M NaCl. Samples were monitored at 260 nm and peaks corresponding to the full-length oligonucleotide species were collected, pooled, desalted on NAP-5 columns, and lyophilized.
[0112]The purity of each oligomer was determined by capillary electrophoresis (CE) on a Beckman PACE...
example 2
Increased Potency of 25mers
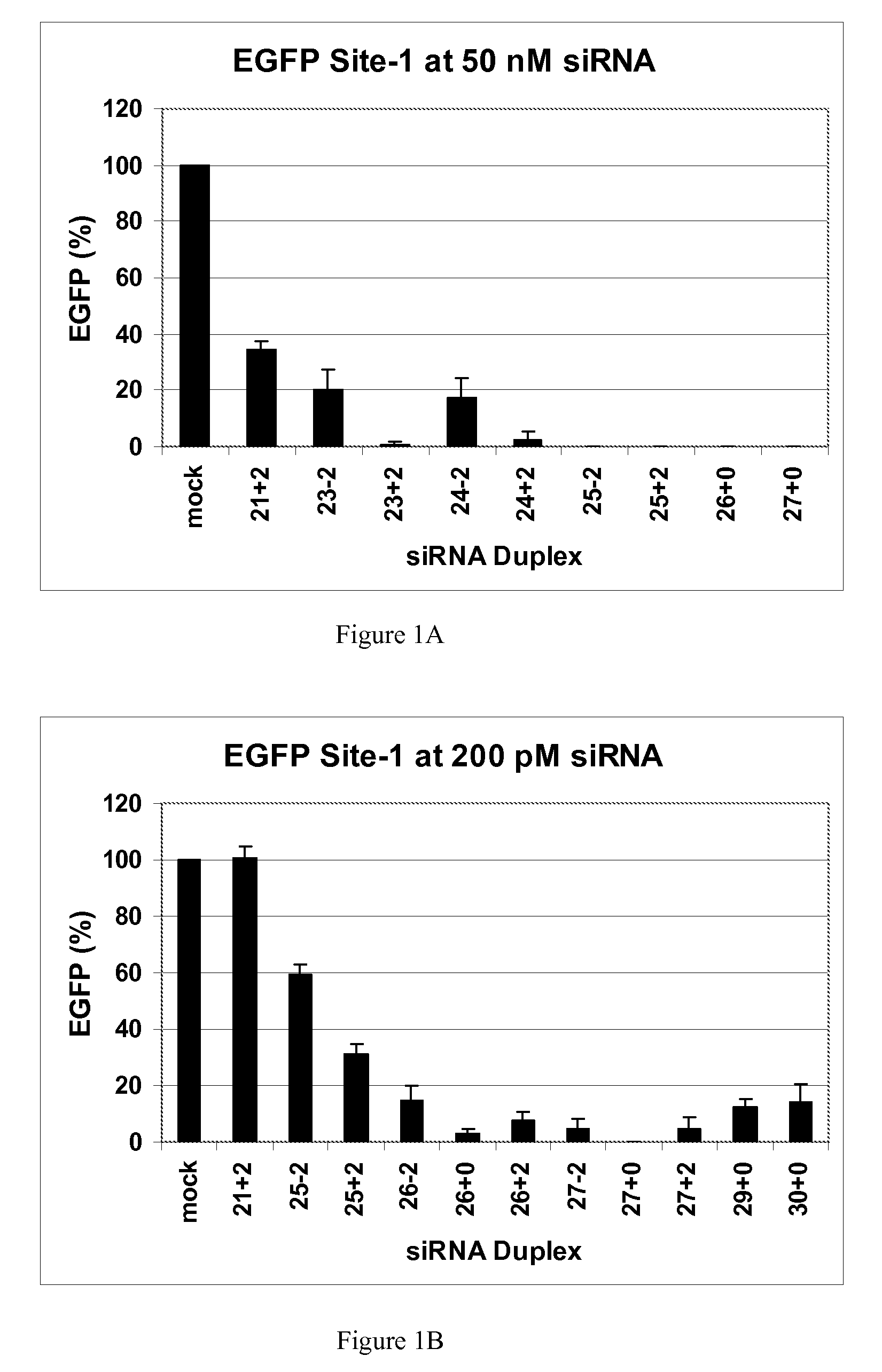
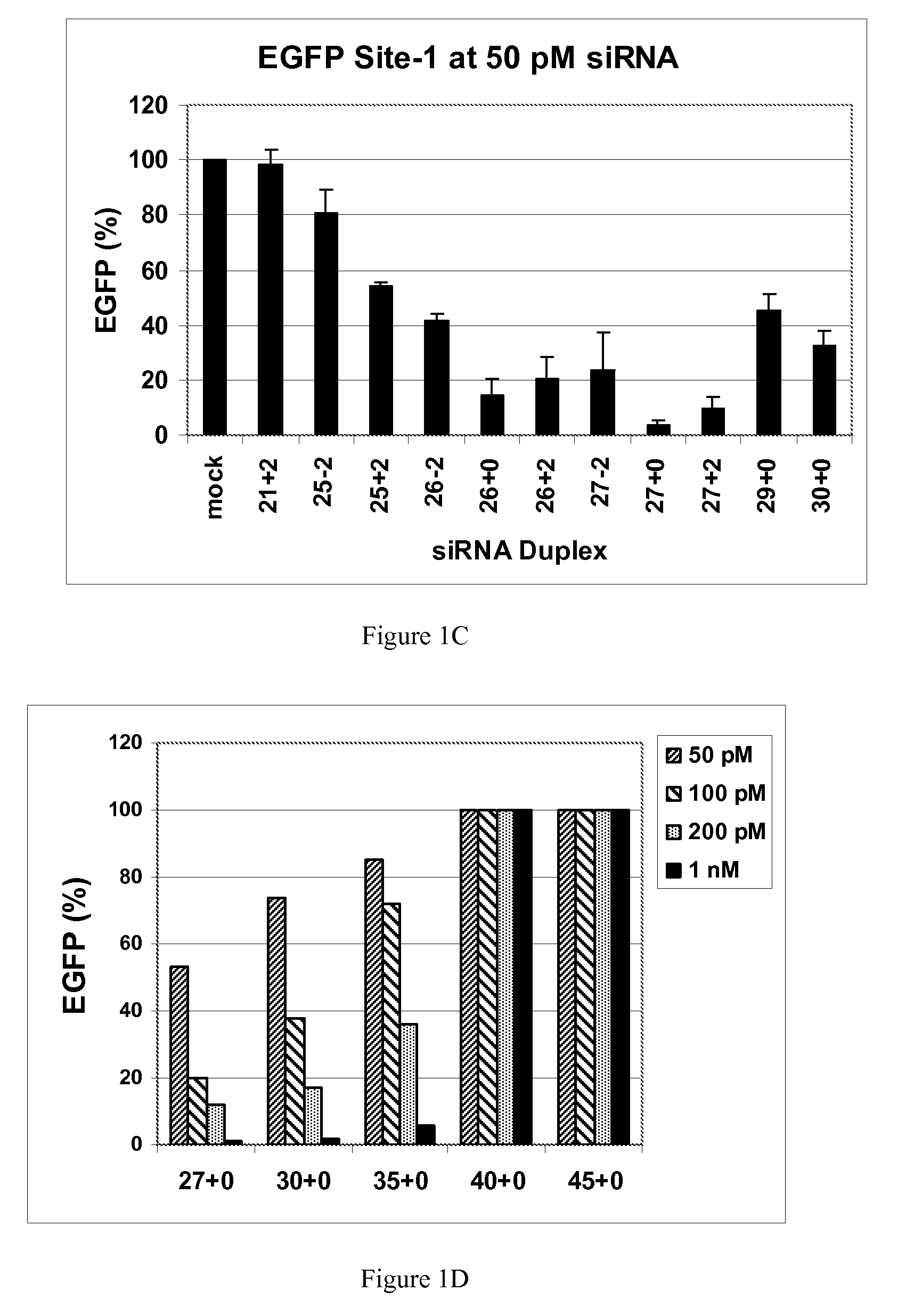
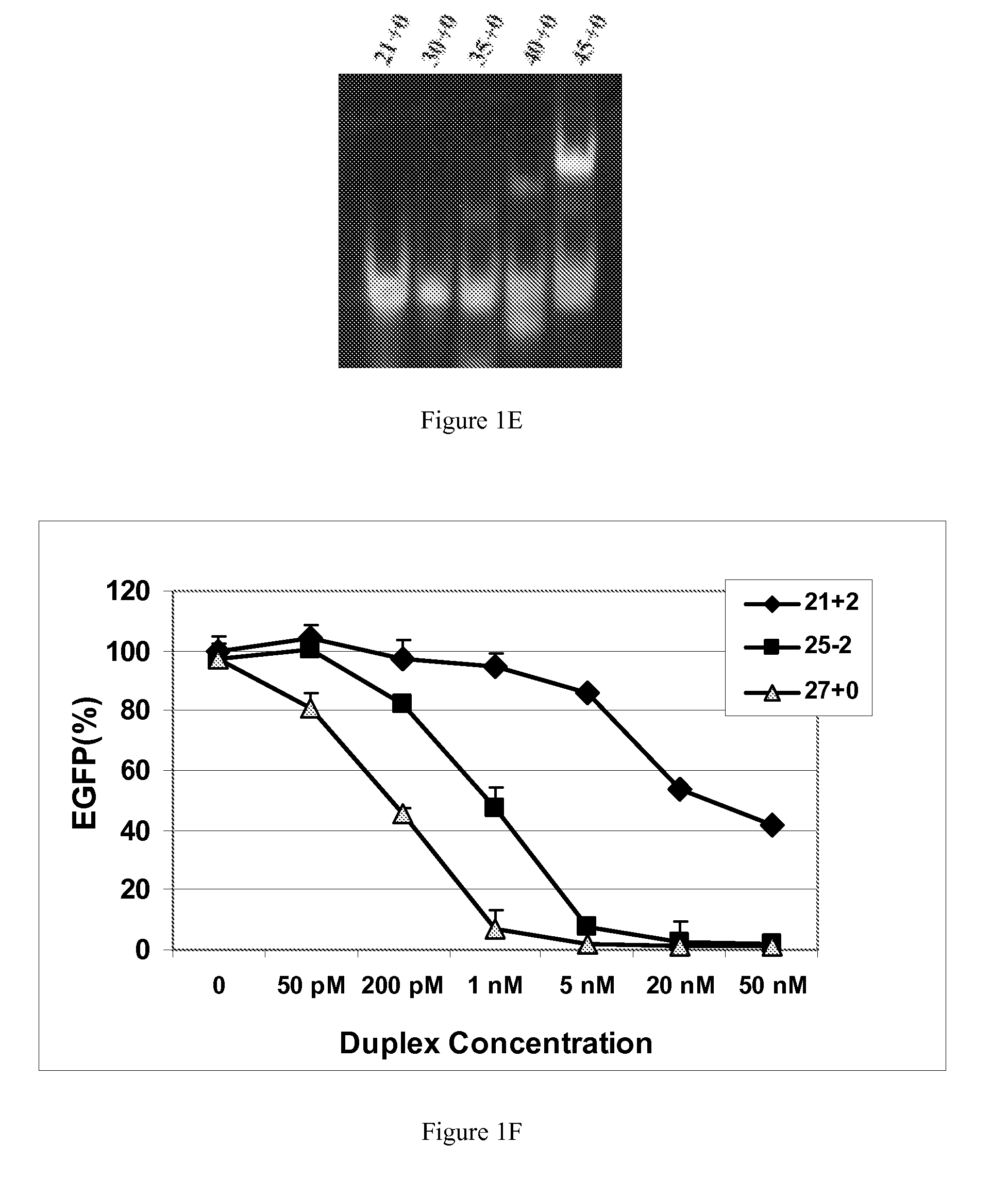
[0117]This example demonstrates that dsRNAs having strands that are 25 nucleotides in length or longer have surprisingly increased potency in mammalian systems than known 21mer to 23mer siRNAs.
[0118]During investigations of the effects of different 5′ and 3′ end structures of dsRNAs made through bacteriophage T7 in vitro transcription (Kim et al., 2004), we observed that some seemed to have greater potency than synthetic 21mer siRNAs directed to the same target site, and that this property seemed to correlate with length. To further explore this phenomenon, we systematically studied the silencing properties of chemically synthesized duplex RNAs of different lengths and designs.
[0119]Cell Culture, Transfection, and EGFP Assays
[0120]HEK 293 cells were split in 24-well plates to 60% confluency in DMEM medium 1 d before transfection. After adding the aliquot of each RNA, 50 μl of Opti Media containing the reporter vectors was added. Next, 50 μl of Opti Media c...
example 3
Dicer Substrates
[0129]This example demonstrates a method for determining whether a dsRNA serves as a substrate for Dicer.
[0130]In Vitro Dicer Cleavage Assays
[0131]RNA duplexes (100 pmol) were incubated in 20 μl of 20 mM Tris pH 8.0, 200 mM NaCl, 2.5 mM MgCl2 with 1 unit of recombinant human Dicer (Stratagene) for 24 h. A 3 μl aliquot of each reaction (15 pmol RNA) was separated in a 15% nondenaturing polyacrylamide gel, stained with GelStar (Ambrex) and visualized using UV excitation.
[0132]Results
[0133]Incubation of 21-bp to 27-bp RNA duplexes with recombinant human Dicer resulted in cleavage of the 23mer, 25mer and 27mer duplexes, but not the 21mer duplex (FIG. 2A). Determinations of relative efficiencies of Dicer cleavage were not possible under the in vitro conditions recommended by the supplier owing to the slow kinetics of this reaction. Aside from the possibility that the dsRNAs longer than 30 bp may need to be preprocessed by Drosha, a micro RNA processing enzyme, to be good ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| inner diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com