Compositions and Methods for Microbe Storage and Delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Freeze-Dried E. coli HU2117; Protocol 1
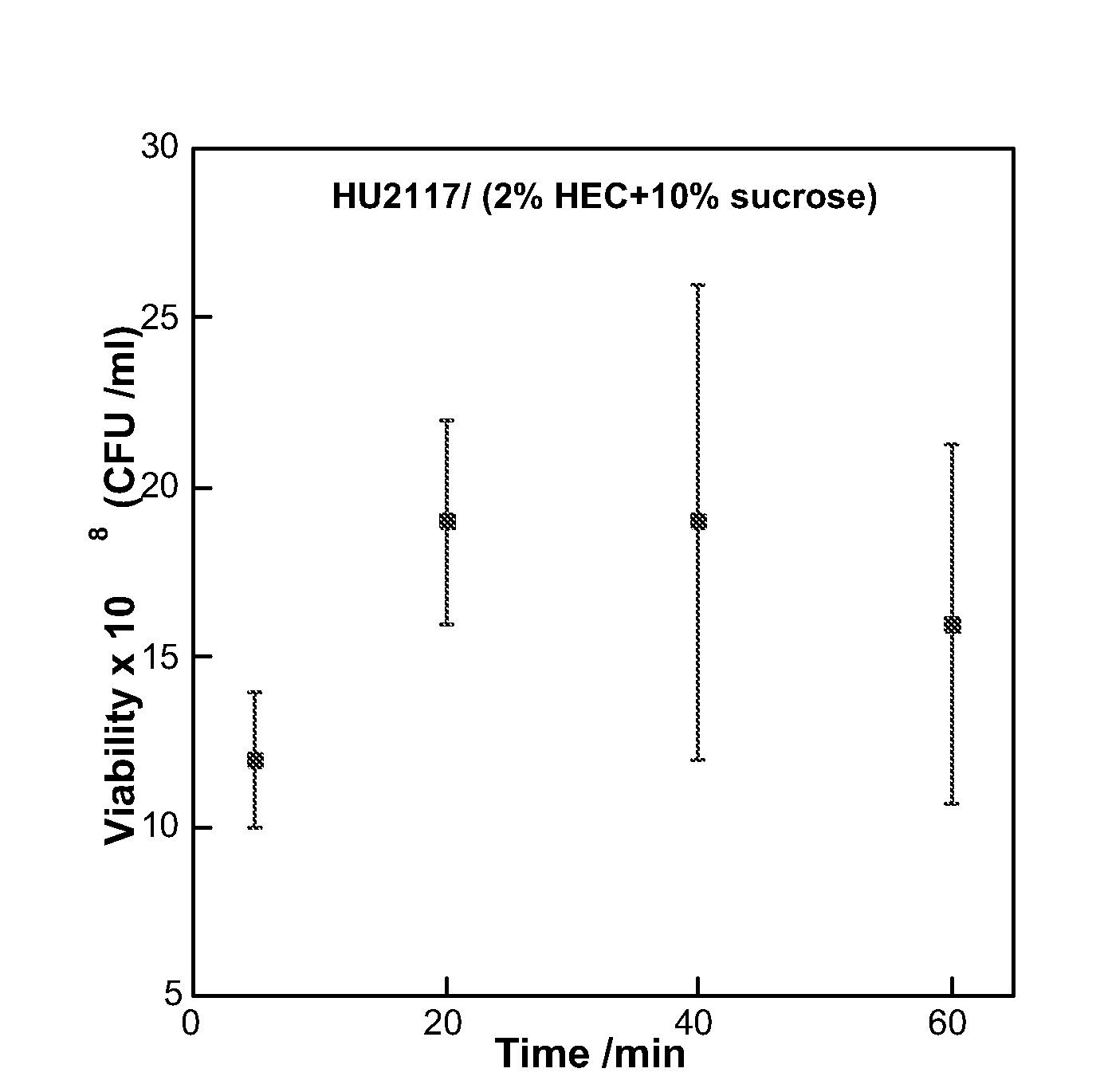
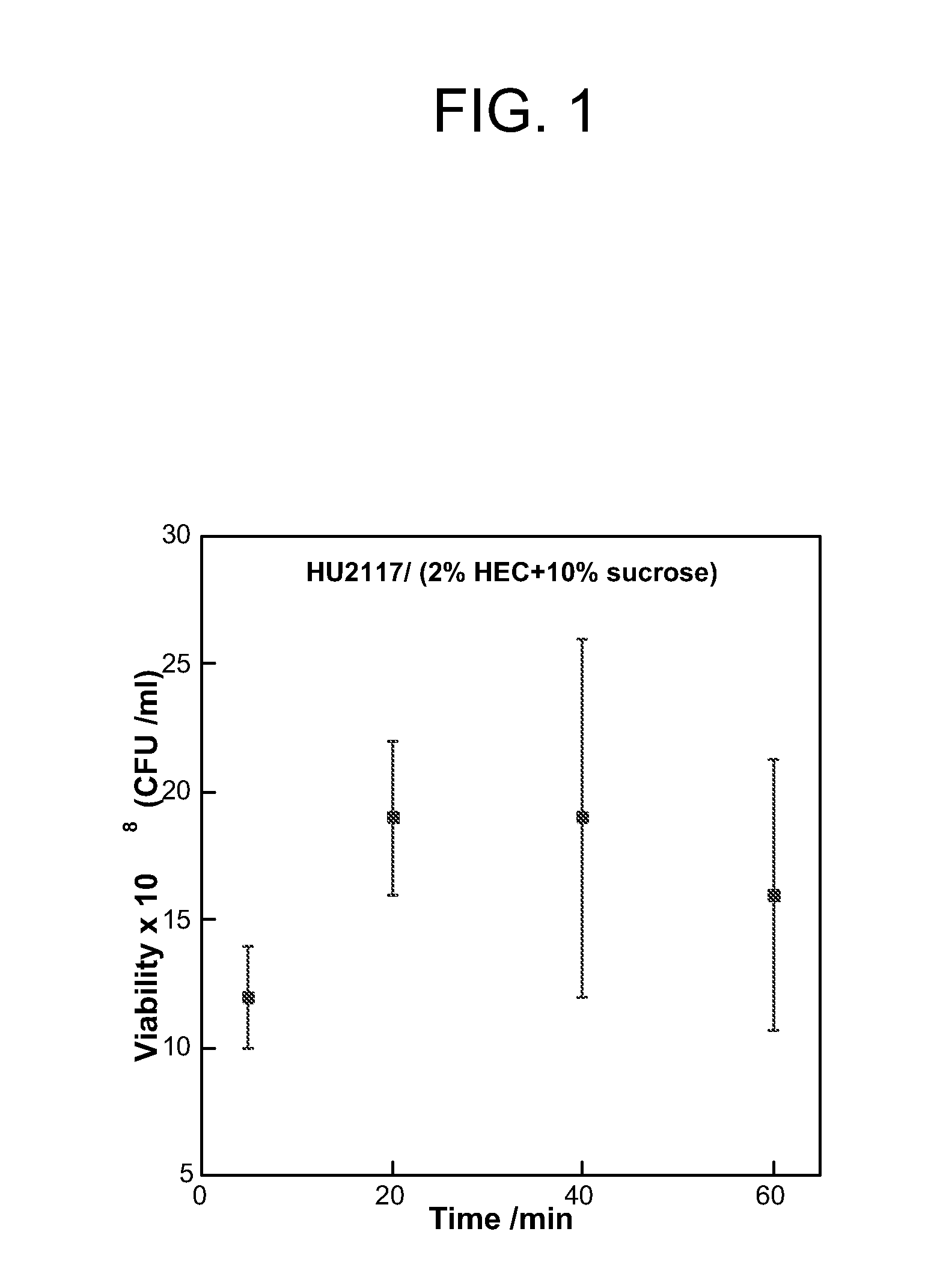
[0120]The goal of the investigation was to examine the effects of different excipients and conditions for lyophilization of HU2117, so as to maintain an effective cell concentration and level of viability of cells freeze-dried in a composition comprising a gelling agent.
Cell Preparation
[0121]Two flasks of cells (Flask A and Flask B) were grown, each from 1 ml of seed stock inoculated into 1 L modified EZ Rich Defined Glycerol medium, incubated at 37±1° C. for 8 hrs with constant shaking at 250 RPM. At the end of 8 hours, the OD600 of Flask A was 2.53 and the OD600 of Flask B was 1.11.
[0122]The cells were collected by centrifugation at 4° C., at 6000 RPM for 8 min. The pelleted cells were washed twice with 0.9% saline and once with 10 mM citrate buffer, pH 7.0. The cells pelleted from each liter of culture were resuspended into 2-3 ml of 10 mM citrate buffer, pH 7.0, for a final volume of approximately 5 ml.
[0123]The concentratio...
example 2
Preparation fo Freeze-Dried E. coli HU2117; Protocol 2
Cell Preparation
[0143]Two flasks of cells (Flask A and Flask B) were grown, each from 1 ml of seed stock inoculated into 1 L Modified EZ Rich Defined Glycerol medium, incubated at 37±1° C. for 8 hrs with constant shaking at 250 RPM. At the end of 8 hours, the OD600 of Flask A was 1.89 and the OD600 of Flask B was 1.53.
[0144]The cells were collected by centrifugation at 4° C., at 6000 RPM for 8 min. The pelleted cells were washed twice with 0.9% saline and washed once with 10 mM citrate buffer, pH 7.0.
[0145]The cells pelleted from each liter of culture were resuspended into 2-3 ml of 10 mM citrate buffer, pH 7.0, for a final volume approximately 5 ml.
[0146]The concentration of the resuspended cells was determined using plate counts. Resuspended cells from Flask A had a viable cell concentration of 1.2±0.3×1011 CFU / ml, and resuspended cells from Flask B had a viable cell concentration of 7.7±0.3×1010 CFU / ml. The cells from Flask A ...
example 3
Preparation of Freeze-Dried E. coli HU2117; Protocol 3
Cell Preparation
[0169]One 2 liter flask of cells was grown from 1 ml of seed stock inoculated into 1 L Modified EZ Rich Defined Glycerol medium, incubated at 37±1° C. for 8 hrs with constant shaking at 250 RPM. At the end of 8 hours, the OD600 was 2.2±0.03.
[0170]The cells were collected by centrifugation at 4° C., at 6000 RPM for 8 min. The pelleted cells were washed twice with 0.9% saline and washed once with 10 mM citrate buffer, pH 7.0.
[0171]The pelleted cells were resuspended into 2-3 ml of 10 mM citrate buffer, pH 7.0, for a final volume of approximately 10 ml.
[0172]The concentration of the resuspended cells was determined using plate counts. The resuspended cells had a viable cell concentration of 4.9×1010 CFU / ml.
Lyophilization
[0173]For each test, 0.5 ml of resuspended cells were mixed with 1.5 ml of an excipient selected from the list below and 10 ml of 2% autoclaved hydroxyethyl cellulose (HEC).[0174]Excipients (shown as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com