Methods, Devices, Kits and Systems for Defunctionalizing the Gallbladder
a gallbladder and kit technology, applied in the field of methods, devices, kits and systems for defunctionalizing the gallbladder, can solve the problems of increasing the risk of forming gallstones, affecting the treatment effect, and patients may elect to suffer from these symptoms for a long tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
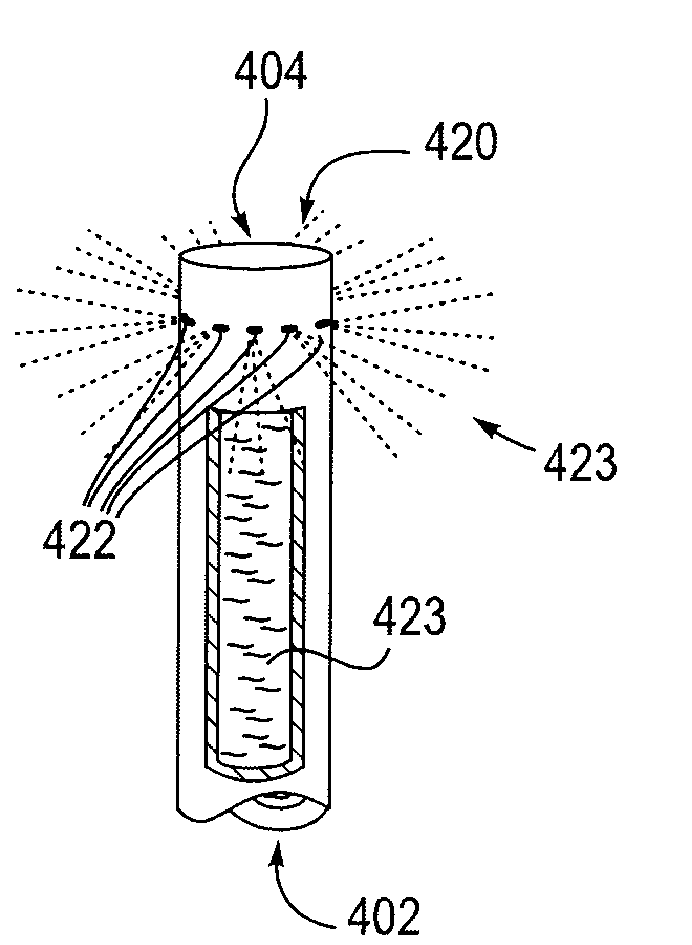
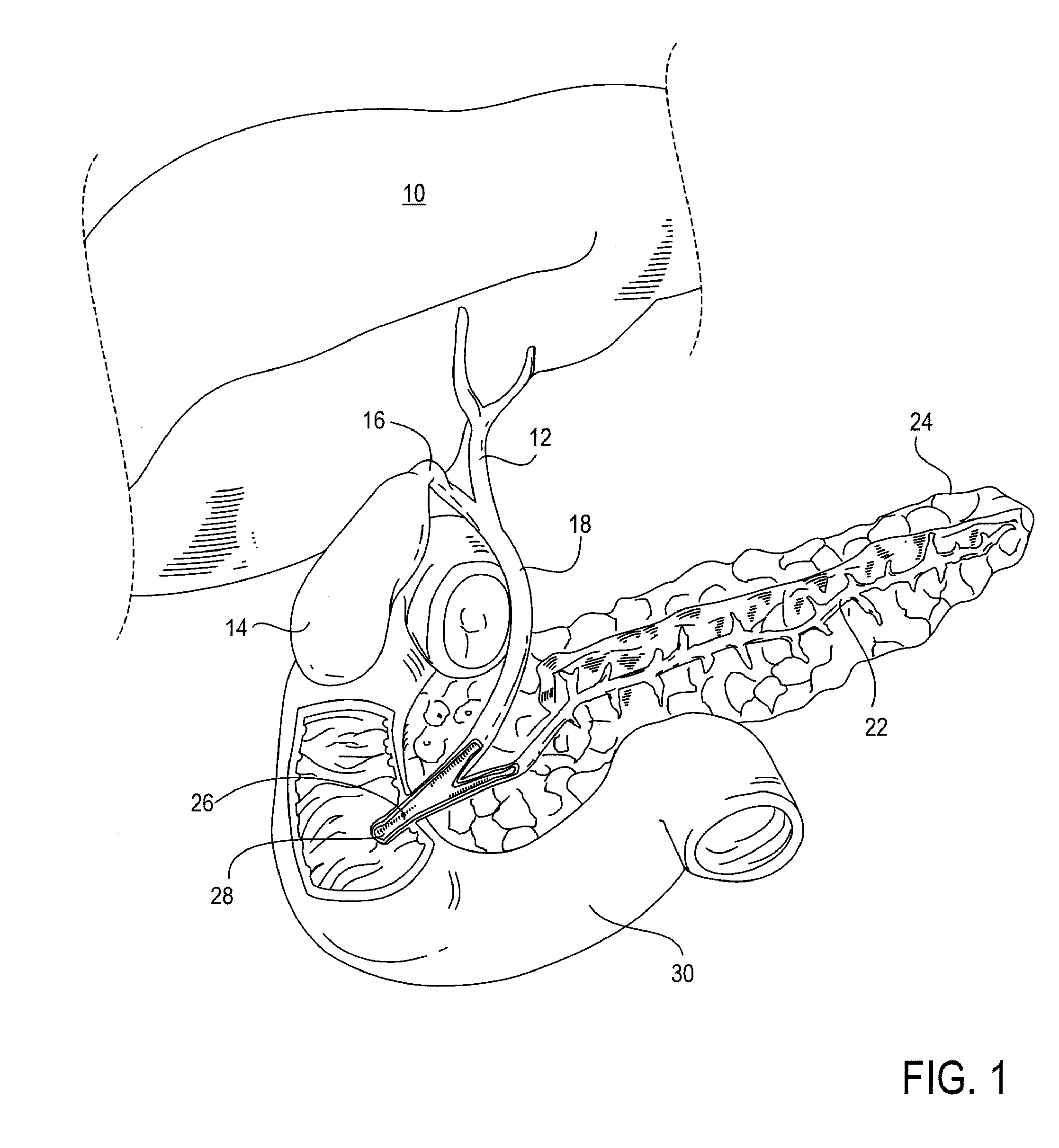
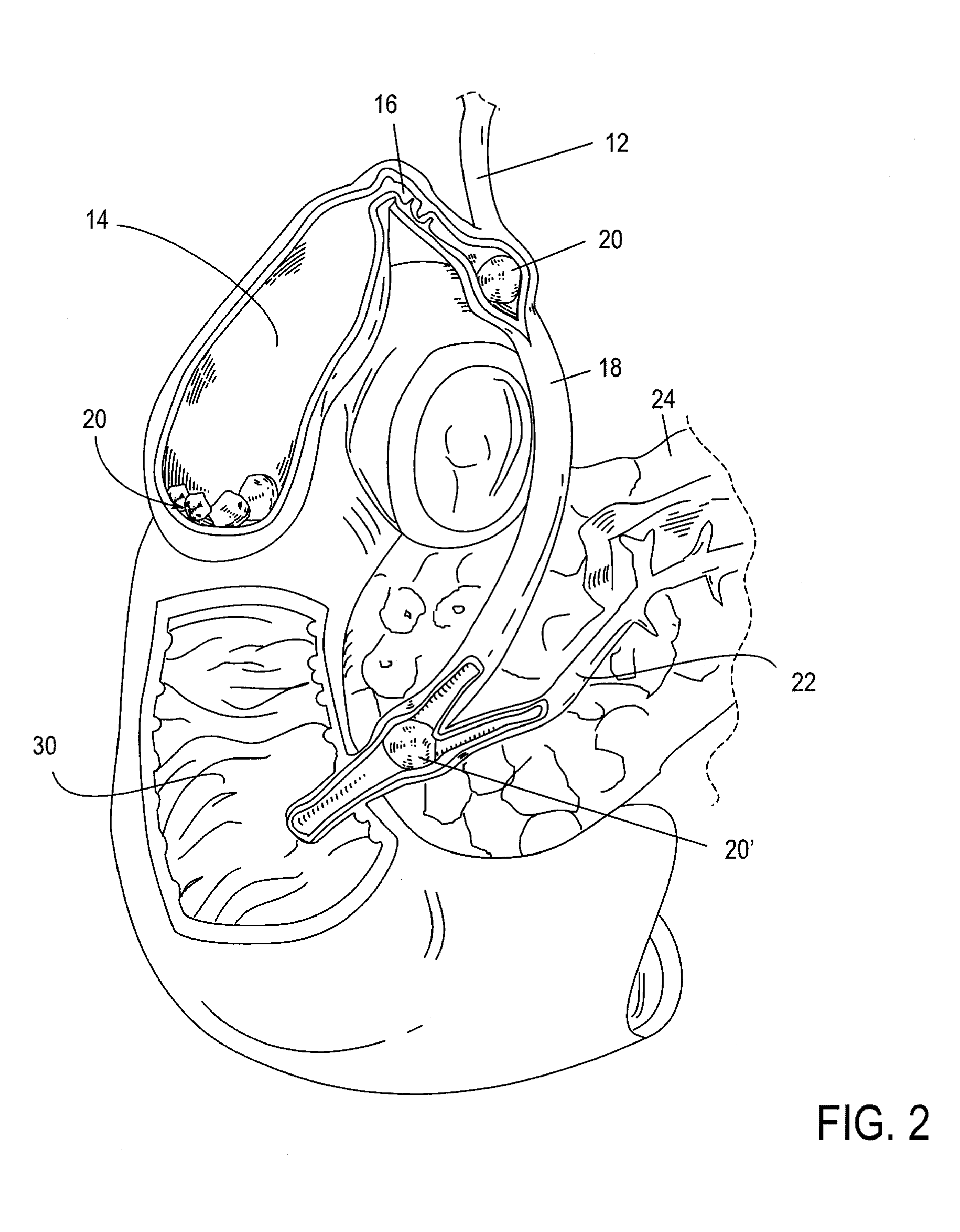
[0035]Devices, systems, methods and kits provided herewith can obviate the need for a plurality of procedures, including, for example: 1) percutaneous cholecystostomy, 2) cholecystectomy, 3) percutaneous trans-hepatic cholangiography (PTHC), and 4) endoscopic retrograde cholangiopancreatography (ERCP). Additionally, disclosed treatment modalities enable treatment of a distal common bile duct 18 obstruction, e.g. secondary to pancreatic carcinoma, cholangiocarcinoma, and / or ampullary carcinoma. As will be appreciated by those skilled in the art, the conventional standard of care for treating biliary disease has been surgical removal of the gallbladder 14 and closure of the cystic duct 16. While this has proven to be an effective mechanism for permanently eliminating biliary disease and its recurrence, the present invention seeks to accomplish the same end in a less invasive and less costly way. This may be achieved by treating biliary disease without requiring the removal of the gall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com