Antibody purification by cation exchange chromatography
a cation exchange chromatography and antibody technology, applied in antibody medical ingredients, immunological disorders, drug compositions, etc., can solve the problems of difficult removal of subcellular fragments, economic purification of proteins, and difficult use as human therapeutics, and achieve the effect of improving the removal of contaminants of chinese hamster ovary proteins (chop)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Purification of a CD20 Antibody
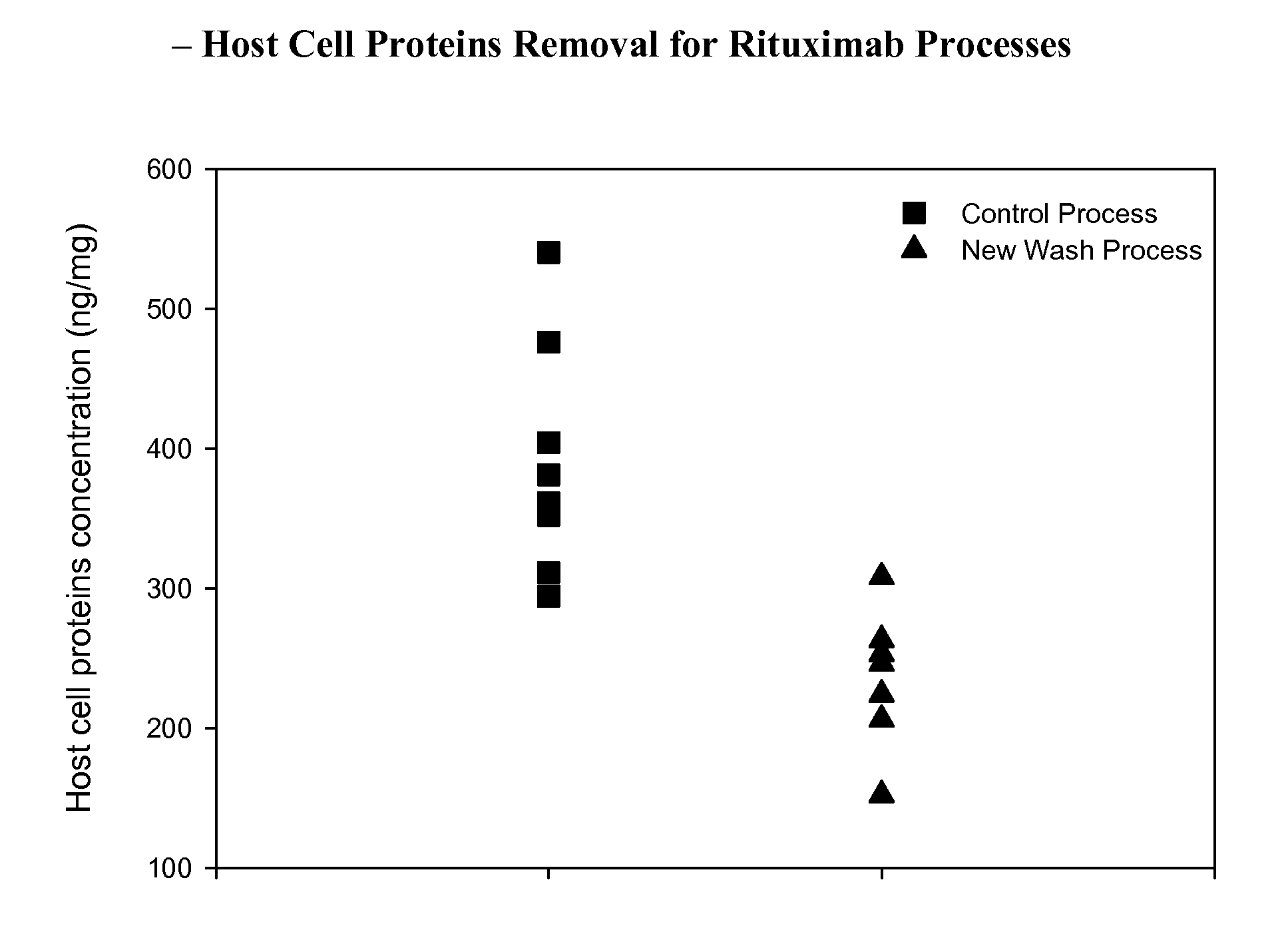
[0116]This example describes an improved cation exchange chromatography process for purifying a CD20 antibody, rituximab. Rituximab is used for therapy of NHL, CLL, RA, MS, etc. The structure of the Rituximab molecule is disclosed in U.S. Pat. No. 5,736,137, Anderson et al., (expressly incorporated herein by reference) as well as FIGS. 1A-1B herein. Rituximab is commercially available from Genentech, Inc.
[0117]Cation-exchange chromatography is used to further reduce the levels of CHOP, DNA, leached protein A, garamycin (GENTAMYCIN®), Rituximab aggregates, and potential viruses. Rituximab binds to the column under the load conditions. The column is then washed, eluted, regenerated / sanitized, and stored until the next use. Multiple cycles may be used to process an entire batch of affinity pool. The cation-exchange pool may be held at room temperature up to 30° C. for up to 3 days or at 5° C. for up to 7 days.
[0118]The cation-exchange resin (POROS 50 HS®,...
example 2
Purification of a VEGF Antibody
[0122]This example describes a cation exchange chromatography process for purifying a recombinant humanized vascular endothelial growth factor antibody (rhuMAb VEGF), bevacizumab. The structure of the bevacizumab molecule is disclosed in U.S. Pat. No. 7,169,901, Presta et al., expressly incorporated herein by reference. See also FIGS. 2A-2B herein. Bevacizumab is commercially available from Genentech, Inc.
[0123]This example summarizes the development studies performed on the cation exchange step for an improved bevacizumab purification process. Three cation exchange resins were evaluated in these studies: CM SEPHAROSE FAST FLOW®, SP SEPHAROSE FAST FLOW® and POROS 50HS®. The cation exchange purification processes using these three resins were evaluated with respect to: process performance (impurities removal, retrovirus removal, and step yield), product quality, process robustness and process fit at all current manufacturing sites. Based on the data gen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conductivity | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com