Rapid magnetic flow assays
a magnetic flow and assay technology, applied in the field of molecular biology and medical science, can solve the problems of easy breakage of molecular tethers, achieve the effect of full antigenicity and binding integrity, rapid methods for forming target specific detection complexes, and wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A) Preparation of Primer Sets
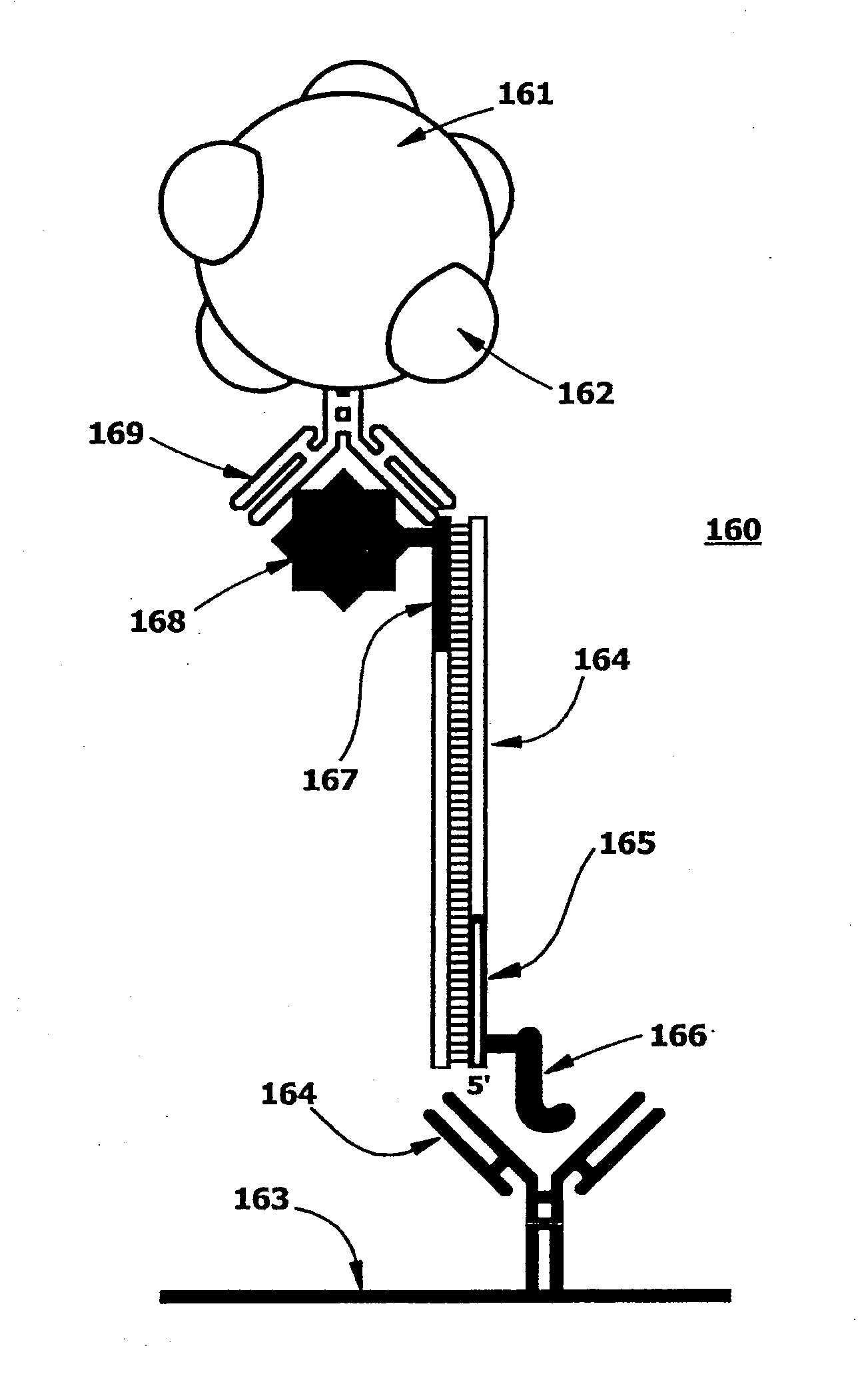
[0189]Reverse primers were first prepared and HPLC purified. Peptides were derivatized with n-terminal hydrazine before use. Oligonucleotides were treated with succinimidyl 4-formylbenzoate in formamide and then reacted with the hydrazine derivatized peptides to form hapten-tagged primers.
[0190]The following peptidyl hapten-tagged primers were used.
5′-Peptidyl OligomersPri-merPrimer Sequence*Peptide Sequence**ACGCCAGTACGATATTCAG(HNA) EQKLISEEDL(SEQ ID NO:1)(NH2) (SEQ ID NO:8)BACCTGGACATCACGGCTTTCAAC(HNA) YPYDVPDYA(SEQ ID NO:2)(NH2) (SEQ ID NO:9)CCCTATTGCAGAGCGAATGAC(HNA) YTDIEMNRLGK(SEQ ID NO:3)(NH2) (SEQ ID NO:10)DTGAACTCCATTAACGCCAGA(HNA) CEEEEYMPME(SEQ ID NO:4)(NH2) (SEQ ID NO:11)ECGACCTGACCAAATGCCAG(HNA) TDFYLK (NH2)(SEQ ID NO:5)(SEQ ID NO:12)FCCTATAACAGCACCCACTATACGG(HNA) DTYRYI (NH2)(SEQ ID NO:6)(SEQ ID NO:13)GCTCTGCGAGCATGGTCTGG(HNA) QPELAPEDPED(SEQ ID NO:7)(NH2) (SEQ ID NO:14)
[0191]These peptide epitopes were selected based on the availability of...
example 2
[0198]PCR amplification was performed in a microfluidic device as follows:
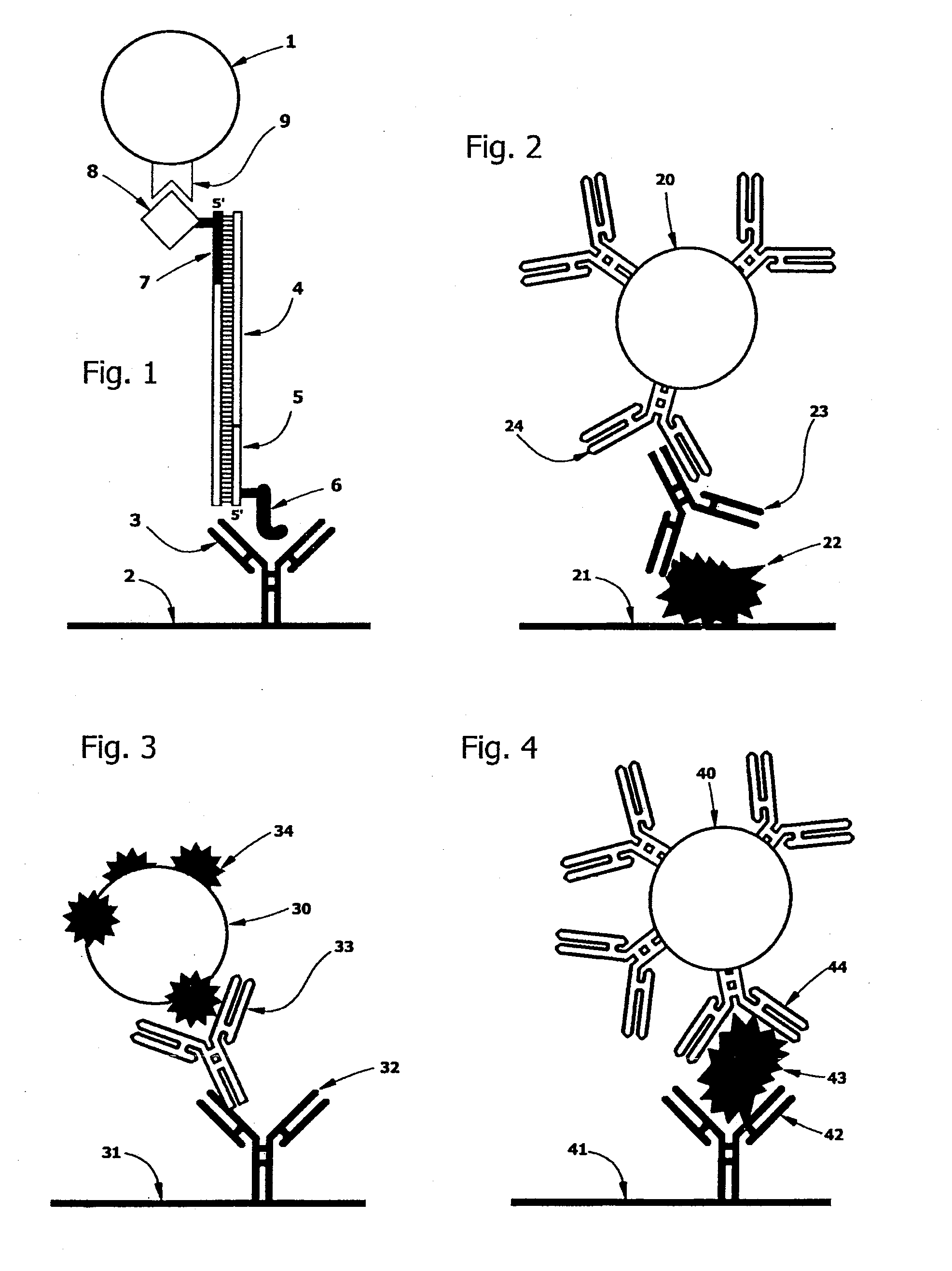
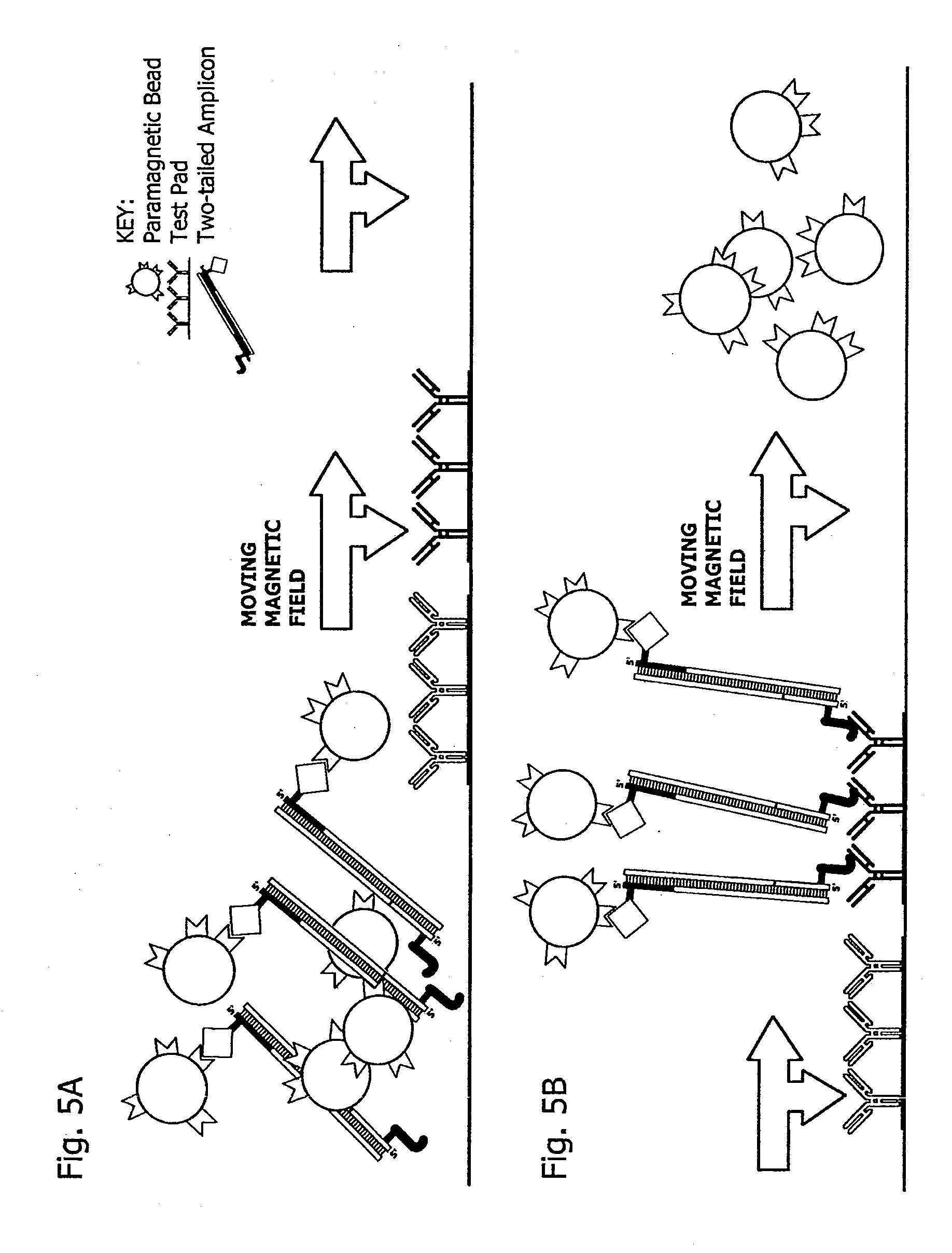
[0199]A microfluidic device was built from stencil-cut laminates. Before final assembly, biotin- and hapten-tagged primer pairs, dATP, dCTP, dGTP and dTTP, TAQ polymerase, and a matrix consisting of TritonX100, BSA, PEG and Trehalose plus magnesium chloride were deposited in the amplification channel or chamber and dried in place under vacuum. Streptavidin-coated magnetic beads (Dynal MyOne Streptavidin Cl, Carlsbad Calif.) were spotted and dried in a chamber adjoining the amplification channels or chambers. Test pad areas in the detection chamber were stenciled (see FIG. 21 for general approach) and gas plasma treated, before antibody solutions were applied and dried in place. Antibody spots were blocked with StabilCoat (SurModics, Eden Prairie Minn.). The device was then treated with a TritonX100:BSA buffer to passivate untreated plastic surfaces.
The following reagents were also prepared:
Lysis Buffer
[0200]4....
example 3
[0222]A result of an assay in which the targets of Example 2 were extracted, amplified and detected is shown in FIG. 6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Area | aaaaa | aaaaa |
| Affinity | aaaaa | aaaaa |
| Paramagnetism | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com