Pro-clotting enzyme, and method for detection of endotoxin or (1-3)-beta-d-glucan using the same
a pro-clotting enzyme and endotoxin technology, applied in the field of nucleic acid fragment encoding a pro-clotting enzyme, can solve the problems of limited standard technique and difficult development of pro-ce activity, and achieve the effects of stable pro-ce activity and quality, low cost, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression of Pro-CE by Use of Insect Cells
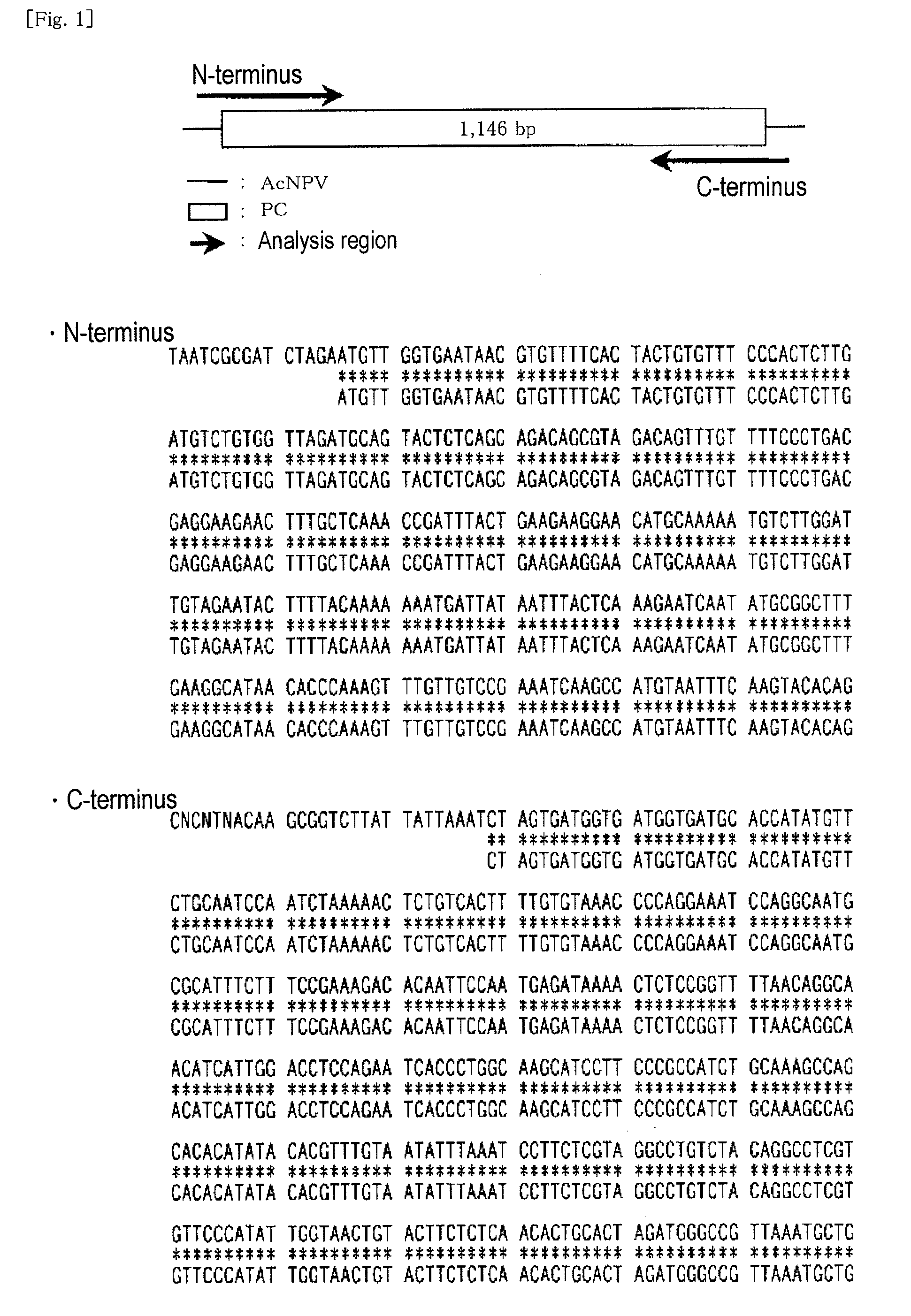
[0162]A cDNA fragment represented by SEQ ID NO: 3 (having a nucleotide sequence of nucleotides 1 to 1125 in SEQ ID NO: 3, a His-Tag sequence being attached to the C-terminus) was kindly offered by Dr. Tatsushi MUTA (Department of Molecular and Cellular Biochemistry, Kyushu University Graduate School of Medical Science; currently, Department of Bio-Science, Tohoku University Graduate School). The cDNA fragment had been prepared through a method disclosed in Non-Patent Document 2. The cDNA fragment was introduced into a transfer vector (pPSC8), and a clone having a predetermined nucleotide sequence was selected. The thus-selected expression vector (PC / pPSC8) DNA fragment and a baculovirus (AcNPV) DNA fragment were co-transfected into Sf9 cells. The virus fluid obtained from the culture supernatant was purified and amplified. The viral DNA fragment was extracted from the cells infected with the baculovirus, and sequenced. Insect cells (expresS...
example 2
Detection of Recombinant Pro-CE Activity
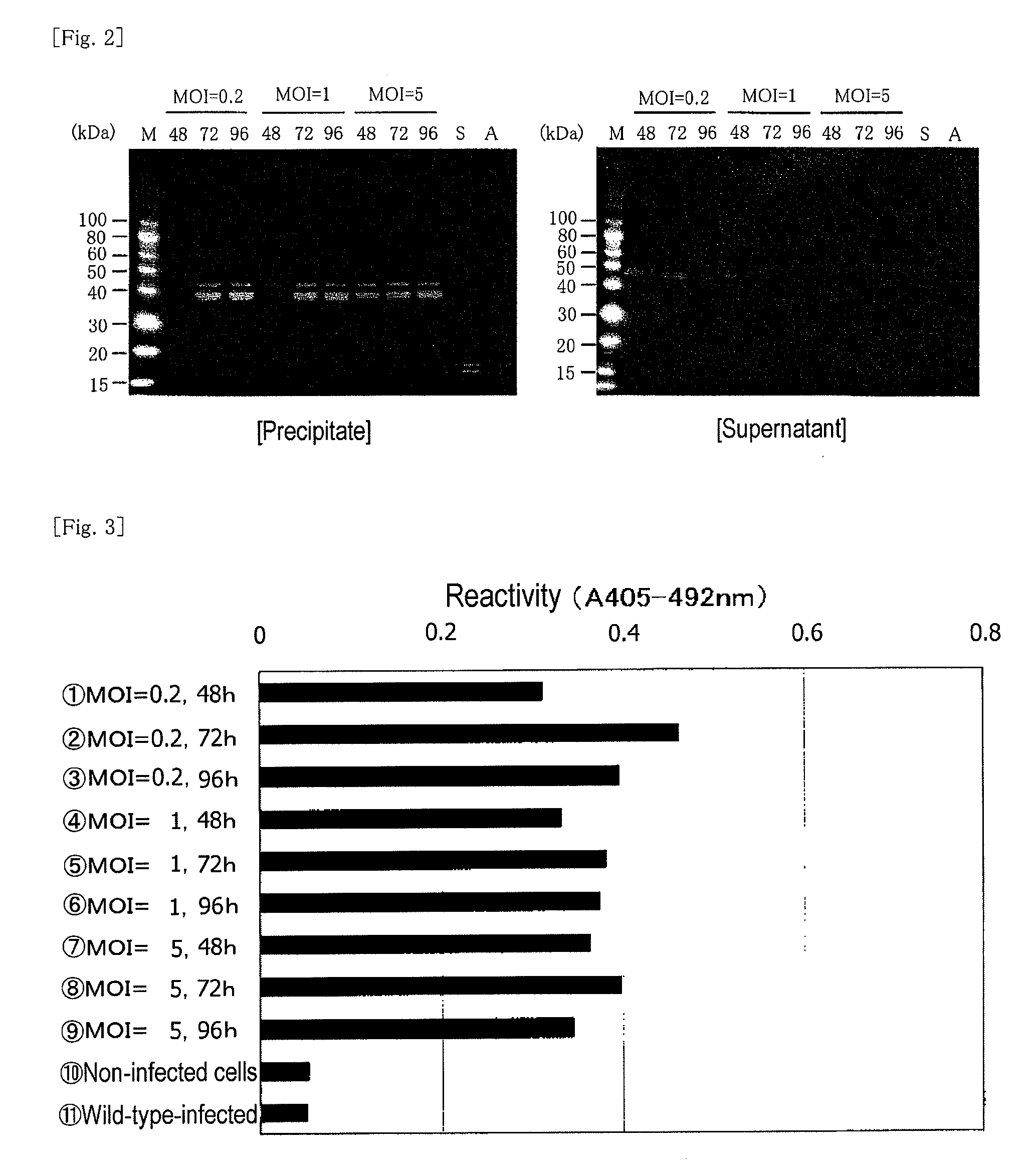
[0182]Insect cells (expresSF+) were diluted with a serum-free Sf-900 II medium so as to adjust the concentration to 1.5×106 cells / mL, and the diluted product (100 mL / per flask) was placed in nine 250-mL Erlenmeyer flasks. The aforementioned third-generation virus fluid was added thereto so as to attain MOIs of 0.2, 1, and 5 (3 flasks each), respectively. Each mixture was subjected to shake cultivation at 130 rpm and 28° C. for 48, 72, and 96 hours. After completion of cultivation, each culture liquid was centrifuged at 3,000×g and 4° C. for 20 minutes, to thereby fractionate into the supernatant and the precipitate. The supernatant was preserved in a frozen state.
Sample 1: MOI=0.2, 48 hours
Sample 2: MOI=0.2, 72 hours
Sample 3: MOI=0.2, 96 hours
Sample 4: MOI=1, 48 hours
Sample 5: MOI=1, 72 hours
Sample 6: MOI=1, 96 hours
Sample 7: MOI=5, 48 hours
Sample 8: MOI=5, 72 hours
Sample 9: MOI=5, 96 hours
Sample 10: non-virus-infected cells
Sample 11: wild-typ...
example 3
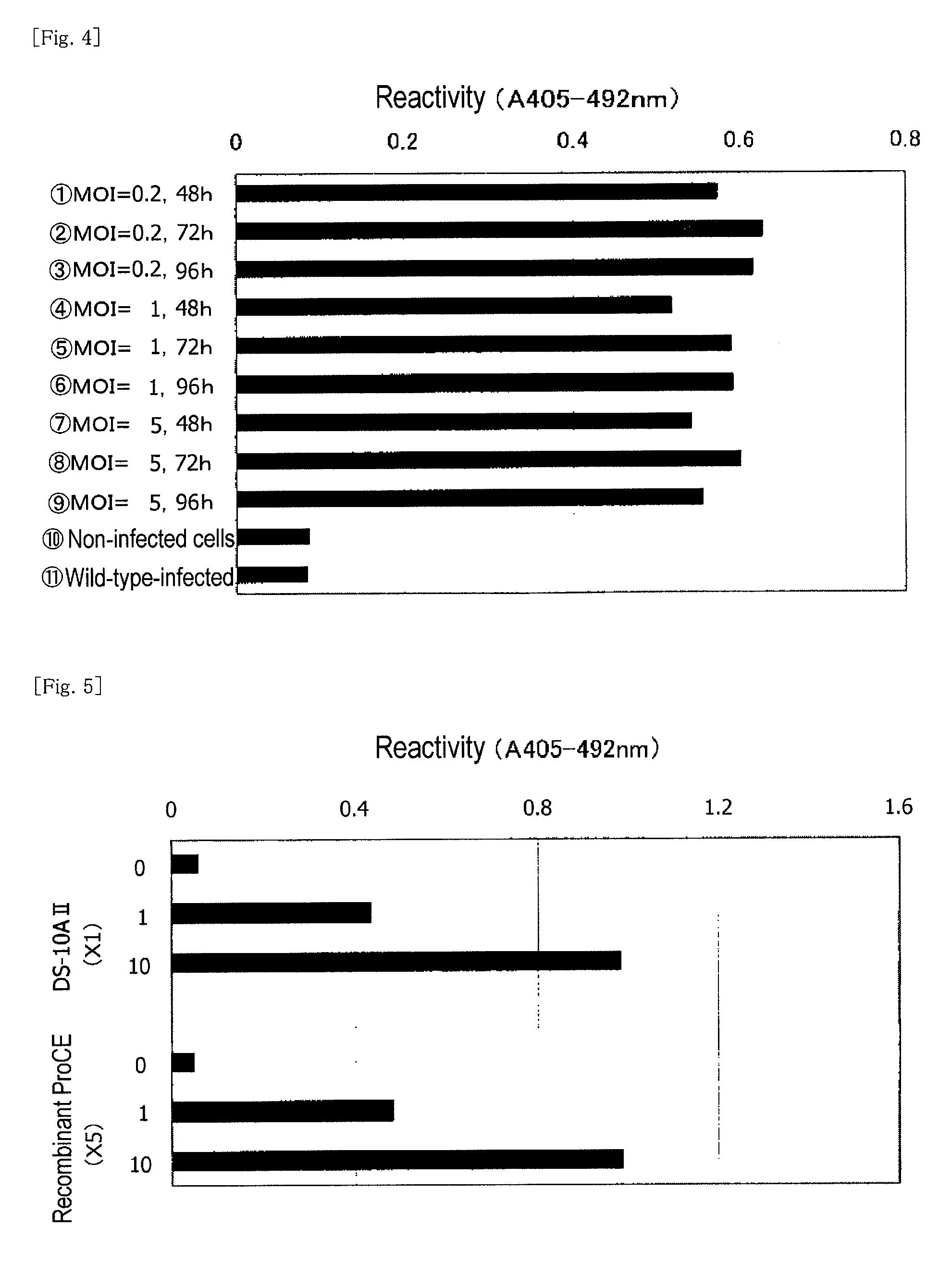
Detection of Expression of Activity in a Complete Reconstitution System Employing Recombinant Pro-CE and Recombinant Factor G
Reagents
[0191](1) recombinant factor G (culture supernatant; α-subunit:β-subunit=2:1)
(2) recombinant Pro-CE (culture supernatant of sample 2 in Example 2)
(3) BG:CSBG (1,495 ng / vial) dissolved in distilled water (1.495 mL), followed by ×10-stepwise dilution
[0192]Each of the culture supernatants (1) and (2), which had been produced through the method disclosed in Japanese Patent Application Laid-Open (kokai) No. 2006-271384 (Patent Document 2), was five-fold diluted with Tris-HCl buffer (pH: 7.5) (50 mL) containing 150 mM NaCl. In this experiment, a nucleotide sequence represented by SEQ ID NO: 21 was employed as a DNA fragment encoding α-subunit of factor G. To the thus-diluted recombinant factor G (25 μL), recombinant Pro-CE (25 μL), Tris-HCl buffer (pH: 8.0) (final concentration: 80 mM), MgSO4 (final concentration: 64 mm), CaCl2 (final concentration: 0.4 mM),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com