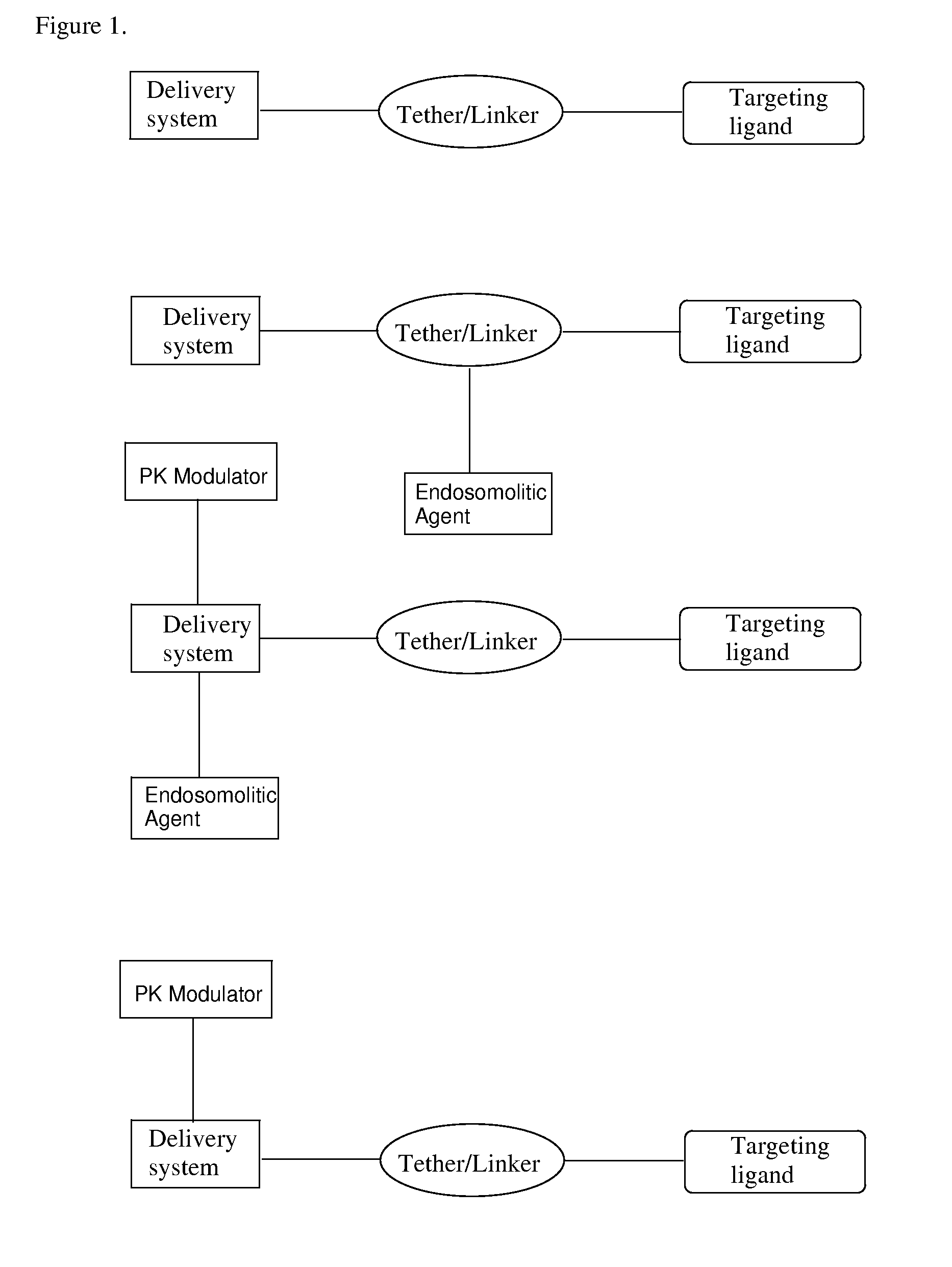
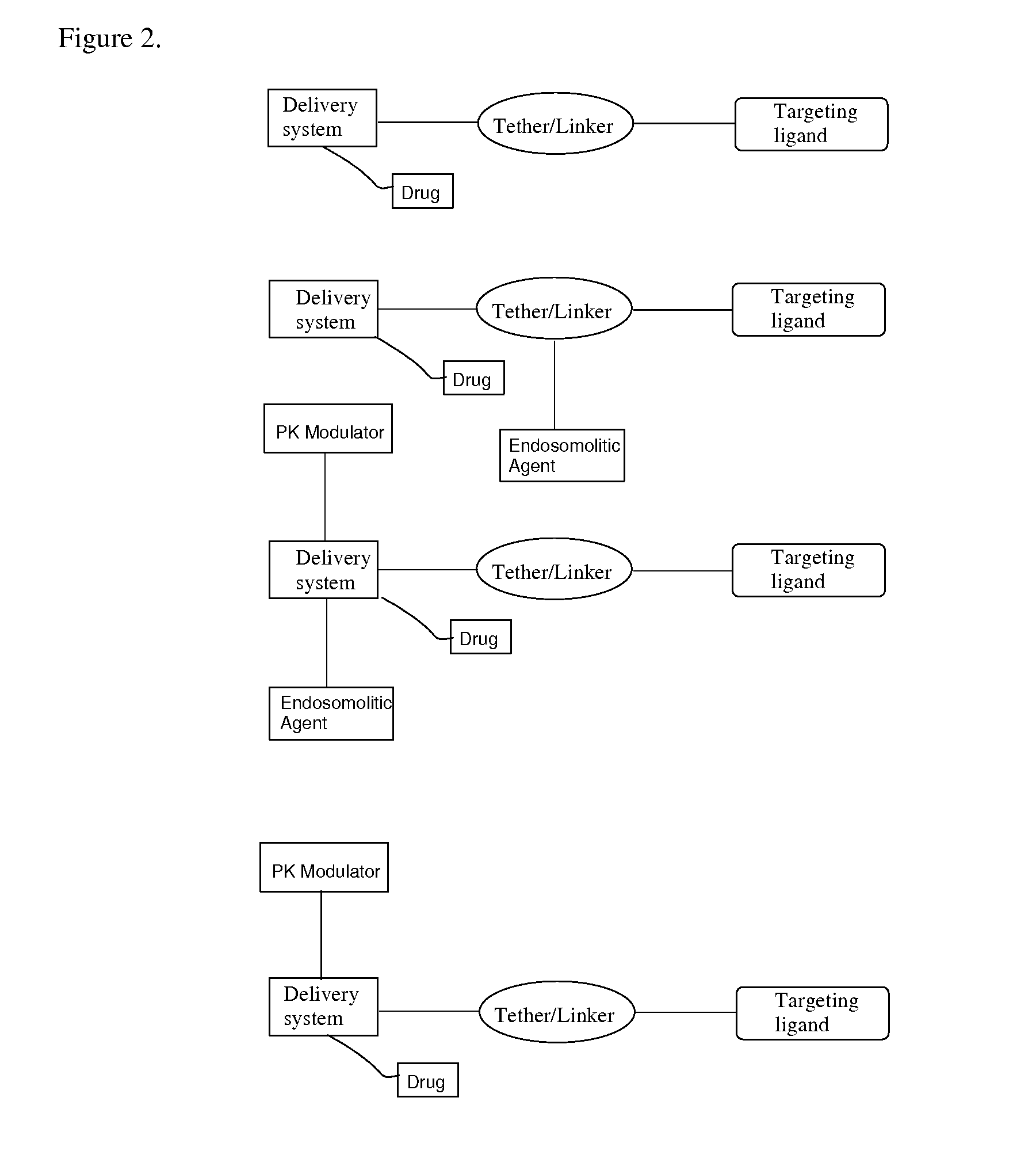
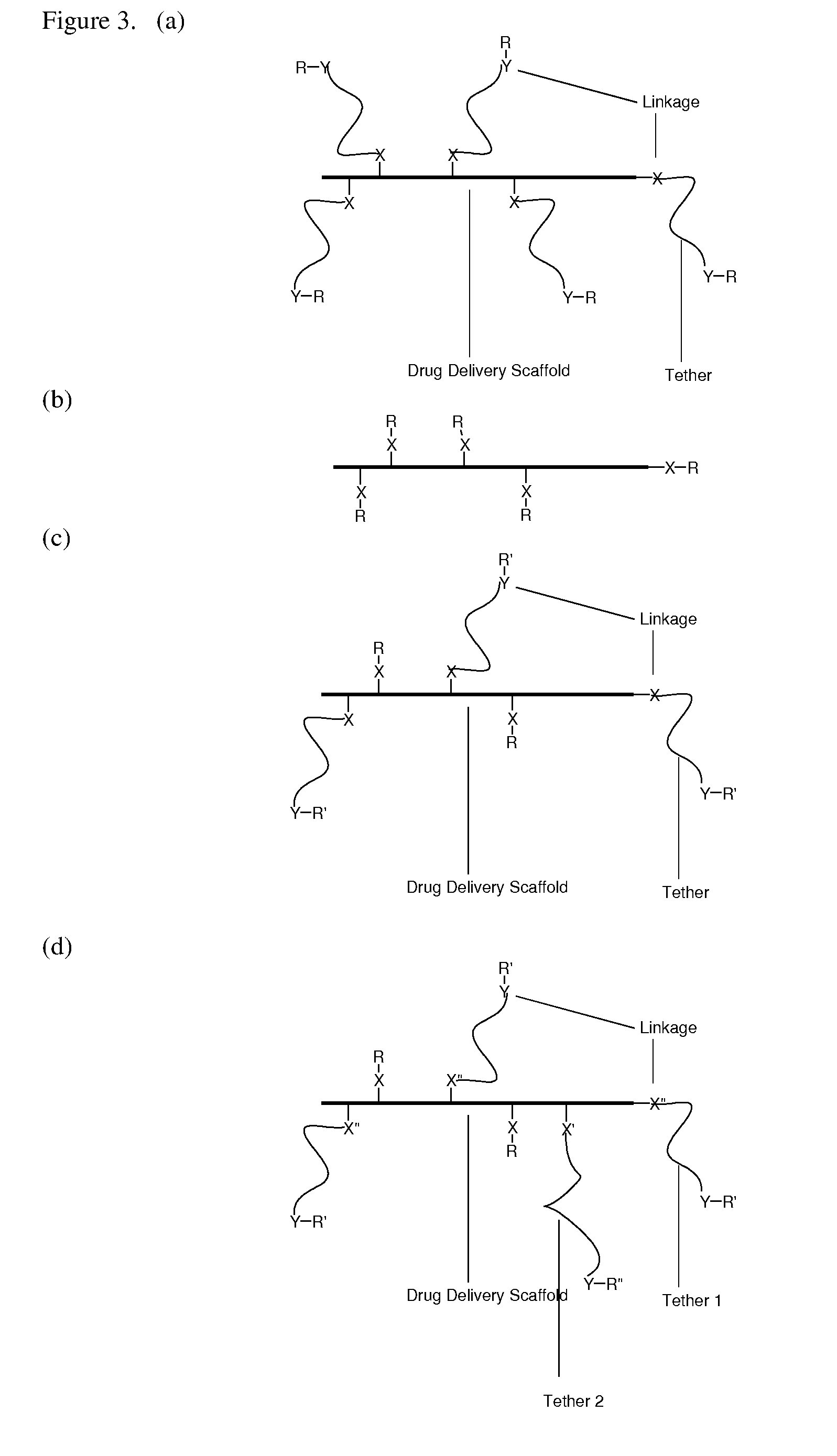
Targeting Lipids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Carbohydrate Building Blocks for Conjugation
[0405]
[0406]Preparation of 101: Galactosamine pentaacetate 100 (52.00 g, 133.63 mmol) was taken in dichloroethane (300 mL) at ambient temperature. TMSOTf (44.55 g, 200.44 mmol) was added that and the mixture stirred at 50 C for 90 minutes in a water bath, heating stopped and the mixture stirred overnight at room temperature. It was poured in to an ice cold sodium bicarbonate solution; extracted with dichloromethane, washed with water and dried over sodium sulfate. Solvents were removed the residue dried under high vacuum overnight to get the compound as dark gum (44.50 g, quantitative). It was used for next reaction with out any further purification. 1H NMR and MALDI confirmed the product formation. MS: Calculated for C14H19NO8, 329.11; Found 352.1 (M+Na).
[0407]Preparation of 102: Compound 101 (43.70 g, 133.56 mmol) and the benzyl ester (41.71 g, 200.34 mmol) were dissolved in dichloroethane (300 mL), molecular sieves (50 g) w...
example 2
Synthesis of Pteroic Acid Precursors for Conjugation
[0438]Appropriately substituted pteroic acid precursor 110 was prepared as follows.
[0439]Synthesis of 4-[(2-isobutyrylamino-4-oxo-3,4-dihydro-pteridin-6-ylmethyl)-(2,2,2-trifluoroacetyl)-amino]benzoic acid 152. To a suspension of pteroic acid (25 g, 61.2 mmol) and DMAP (11.25 g, 92 mmol) in anhydrous pyridine (400 mL), TBDPS chloride (42 g, 153 mmol) was added. The reaction mixture was stirred at room temperature for 30 h after which isobutric anhydride (14.6 g, 92 mmol) was added and the mixture was slightly warmed. An additional 60 mL of pyridine was also added and the reaction mixture was stirred at room temperature overnight. The reaction mixture became homogenous after which pyridine and other volatiles were concentrated in a rotary evaporator. The residue was stirred with EtOAc (1 L) and acetic acid (100 mL) and water (500 mL) for 24 h. The thus obtained slurry was filtered, the residue was washed with water (500 mL), EtOAc (...
example 3
Synthesis of Lipid Conjugates
[0458]
[0459]Preparation of 201: 1, 2-Dioctadecyl sn glycerol (8.50 g, 14.23 mmol) and DSC (5.47 g, 1.5 eq.) were dissolved in DCM (100 mL) and cooled in an ice-water bath. Triethylamine (6.00 mL, 44 mmol) was added and stirred the mixture overnight. The mixture was transferred to a separatory funnel diluted with DCM, washed with bicarbonate solution and water. DCM layer separated and dried over sodium sulfate. Solvents were removed and the residue dried under high vacuum overnight. It was used for the next reaction with out further purification (Yield, 11.50 g).
[0460]Preparation of 202: Compound 201 (4.00 g, 5.42 mmol) and 6-aminohexanoate hydrochloride (1.477 g, 1.5 eq.) were dissolved in DCM and cooled in an ice bath. Pyridine (5 mL) was added to the mixture and stirred overnight. Solvents were removed and the residue dried under high vacuum. The residue extracted with dichloromethane, washed with bicarbonate and water. Crude product was purified by ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
| Degradation properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap