Compositions and Methods for Inducing Hair Growth
a technology of compositions and hair growth, applied in the direction of drug compositions, immunological disorders, metabolism disorders, etc., can solve the problems of high cost, hair loss, and inability to maintain the results achieved, and achieve the effect of reducing the risk of hair loss, and reducing the effect of hair loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0236]Two small-scale human clinical studies were each conducted over a 6 month period to evaluate the potential use of an 0.01% AS101 spray formulation for the prevention of hair shedding and / or enhancing hair growth. Patients received no other alopecia treatment for at least 3 months prior to commencement of the study.
[0237]The sample group consisted of 36 patients, of which 6 withdrew due to non-compliance. Data from a further 14 patients was considered to be unsuitable for analysis with the TrichoScan system, for example, due to untraceable tattoos. 16 patients were therefore available for evaluation.
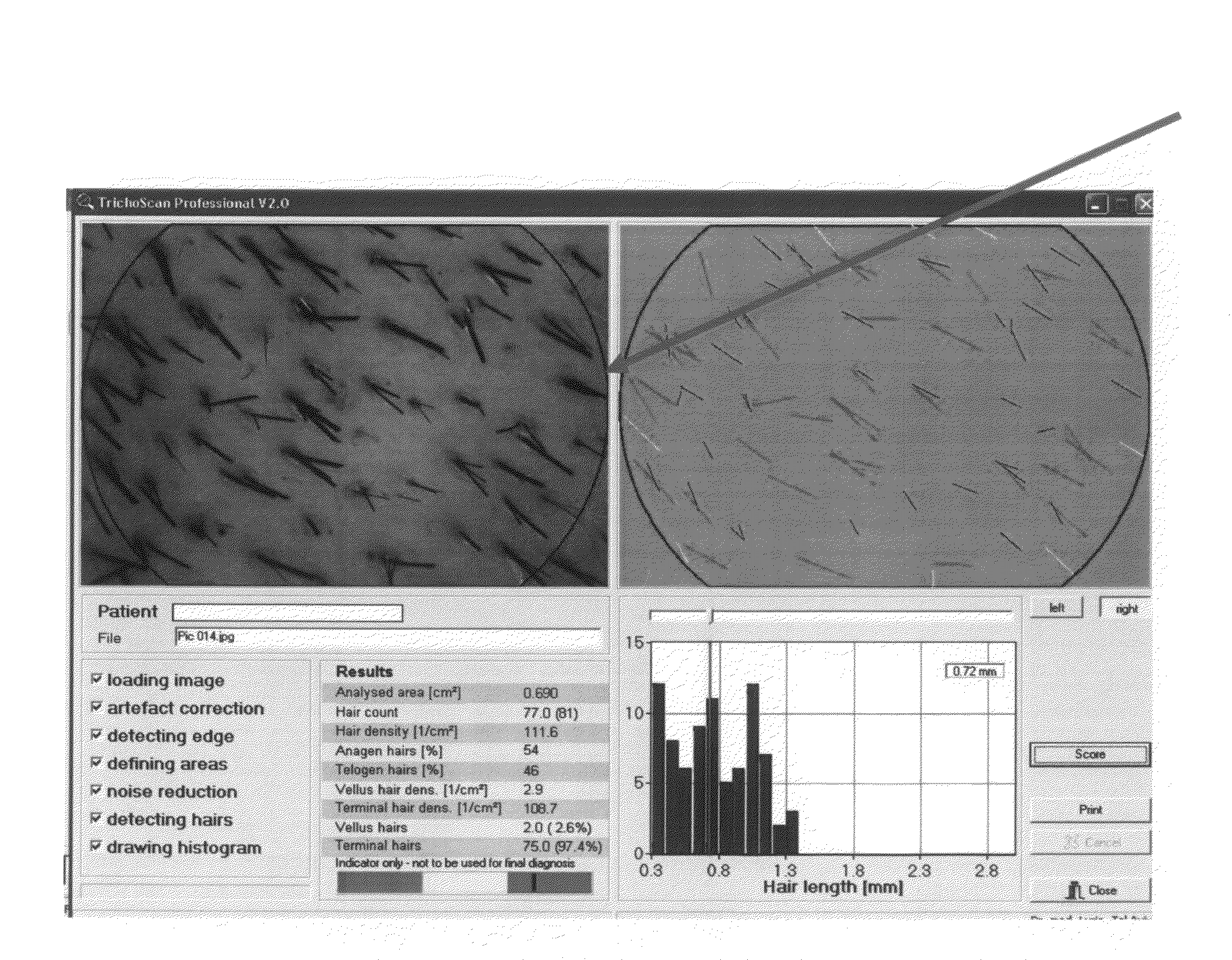
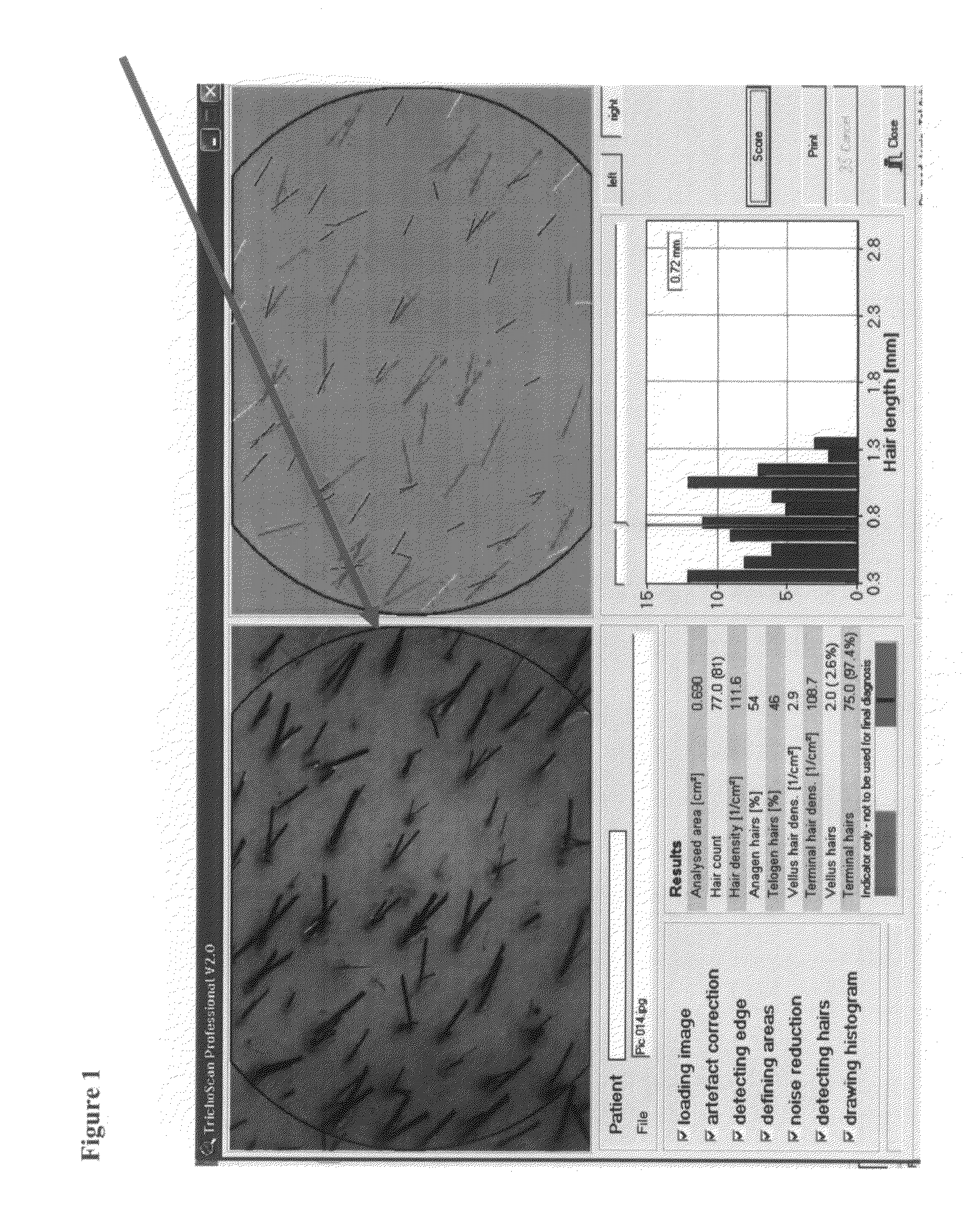
[0238]During an initial visit, a designated area of the scalp (about 0.7 cm2) was shaved. Three days later, the area was tattooed using red ink, and photographed (Nikon digital camera). Patients were provided with a topical spray composition comprising 0.01% AS101 in 20% propylene glycol, 20% water and 60% ethyl alcohol, and were instructed to apply about 2 ml of the composition on ...
example 2
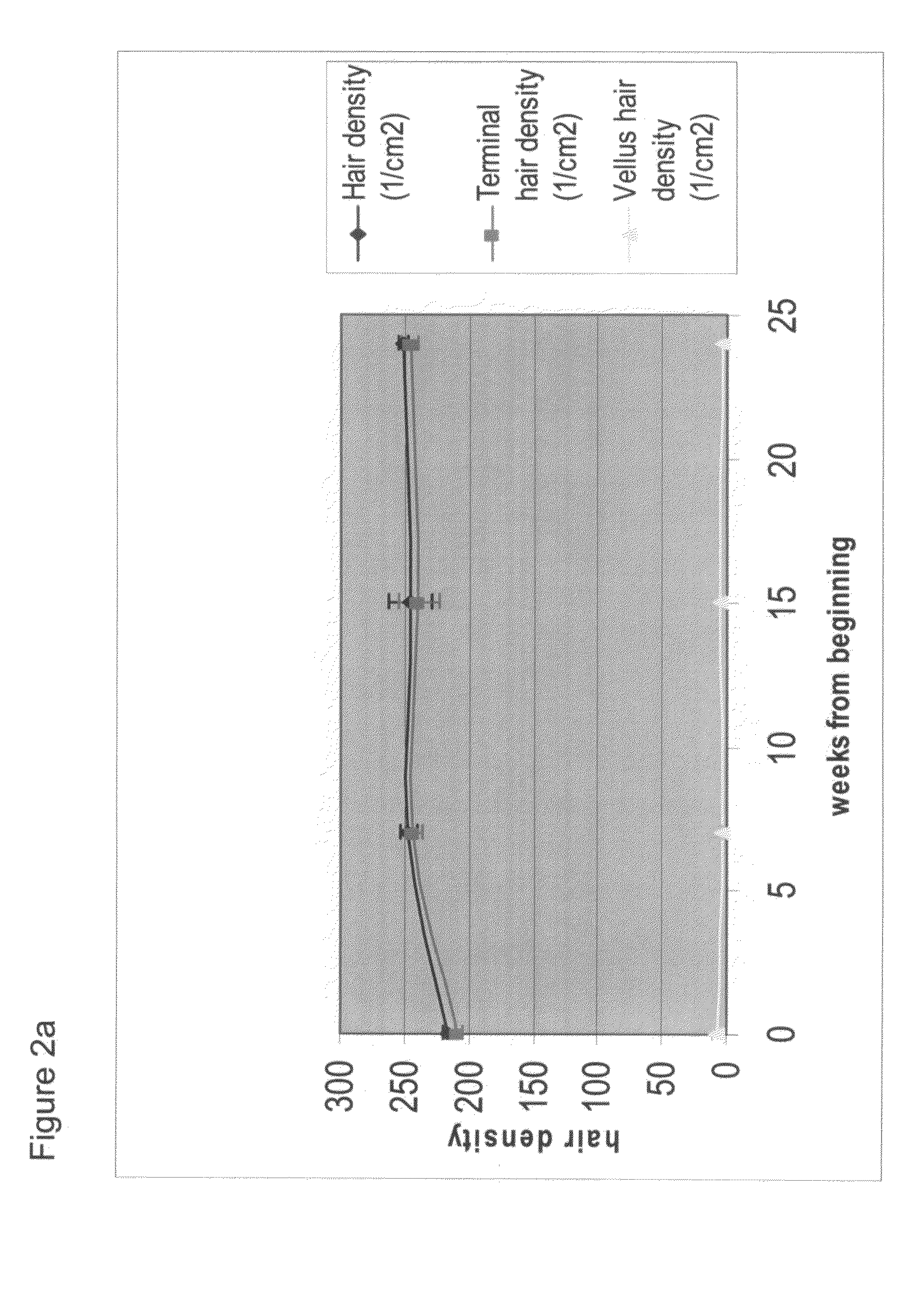
[0244]An individual patient, a woman over the age of 60 applied a spray containing 0.01% AS101 in 20% propylene glycol, 20% water and 60% ethyl alcohol twice daily. The results obtained, as presented in FIG. 3, show a significant increase in the three categories of hair density studied, which continued for the 6 month period of the trial. It is therefore demonstrated that a dosage regime of twice daily administration of AS101 results in increased hair density in a subject suffering from female pattern baldness.
example 3
[0245]A patient suffering from alopecia induced by an autoimmune disease applies a spray containing 0.01% AS101 in 20% propylene glycol, 20% water and 60% ethyl alcohol twice daily. The results are monitored at commencement of the study, after about 1 month, after about 3 months, and after about 6 months. Hair density, terminal hair density, and vellus hair density are analyzed, using the DatInfR Image DB image archiving system, and TichoScan software.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com