High concentration antibody and protein formulations
a protein and formulation technology, applied in the field of high concentration formulations of antibodies, can solve the problems of unsuitable liquid formulations, difficult further processing, and difficult to obtain, and achieve the effects of high concentration of protein or antibody formulations, stable, and low viscosity and turbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Anti-IgE rhuMAbE25 (“E25”) Formulation
[0270]Formulations of the monoclonal anti-IgE antibody rhuMAbE25 were prepared from E25 bulk residual Lot K9094A (40 mg / ml rhuMAb E25, 85 mM trehalose, 5 mM histidine, pH 6, 0.01% Tween 20) or rhuMAbE25 Q-Pool (5 mg / ml rhuMAb E25, 25 mM Tris, 200 mM NaCl).
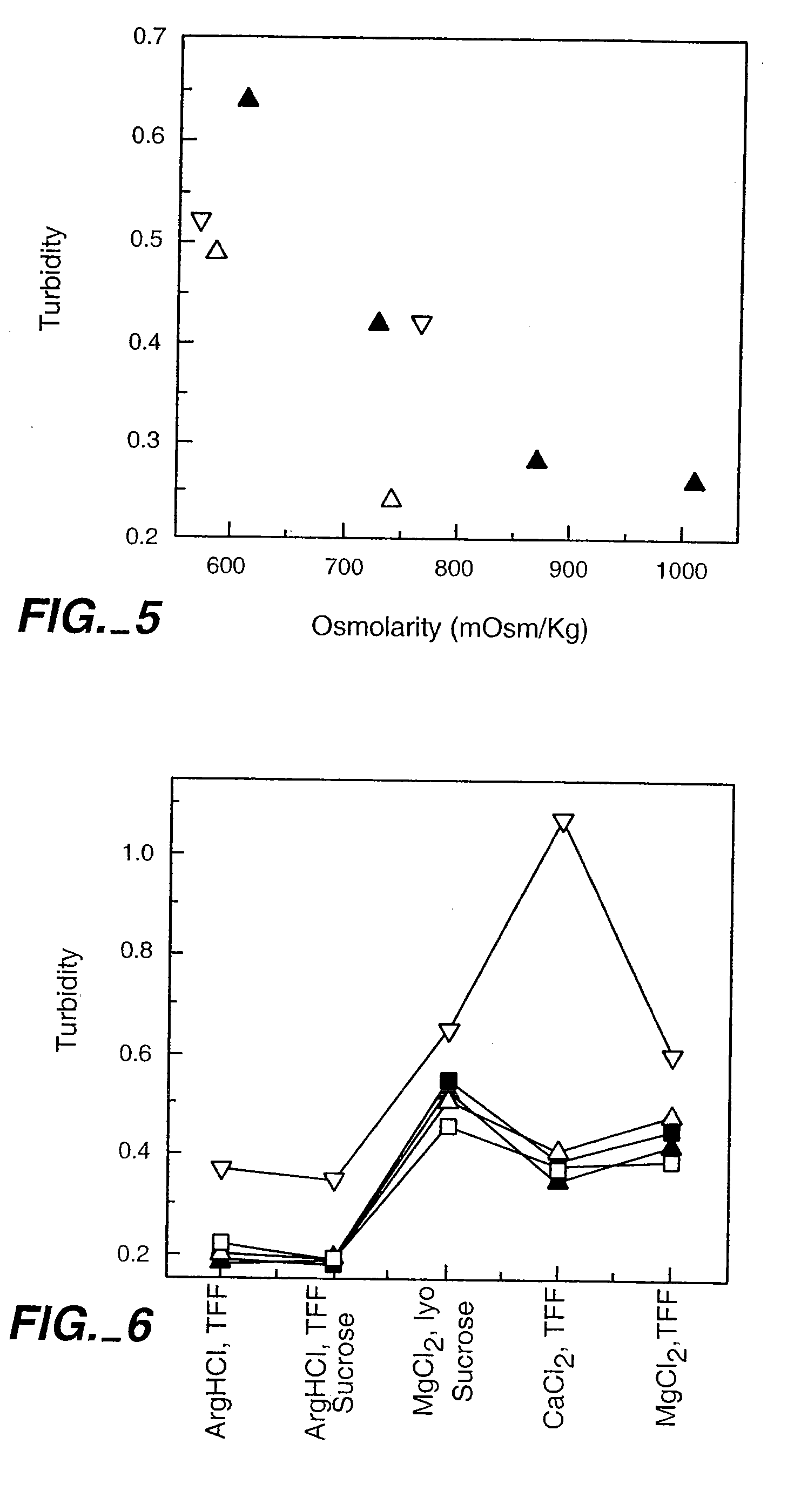
[0271]Aqueous solutions of rhuMAbE25 was prepared by dialysis into different buffers (20 mM His-HCl and 200 mM Arg-HCl, pH 6.0) at 2-8° C. using a Slide-A-Lyzer Dialysis Cassette (Pierce). The samples were then transferred into the sample reservoir of a Centricon-30 centrifugal microconcentrators (Amicon). The proteins were concentrated by spinning the Centricon-3 concentrator at 4000-5000 g until the desired protein concentration is achieved.
[0272]The samples were then concentrated to ˜150 mg / ml of rhuMAb E25 using ultrafiltration. Tween 20 was added to each preparation to a final concentration of 0.02%. All formulations were filtered, aseptically filled into 3 cc FormaVitrum vi...
example 2
Method and Materials
[0273]Stability Studies All formulations were filled at 1 ml in 3 cc FormaVitrum glass vials and stoppered with 13-mm Diakyo stoppers in a Class 100 sterile filling suite. Vials were placed at −70, 2-8, 15, 30 and 40° C. in light impermeable containers.
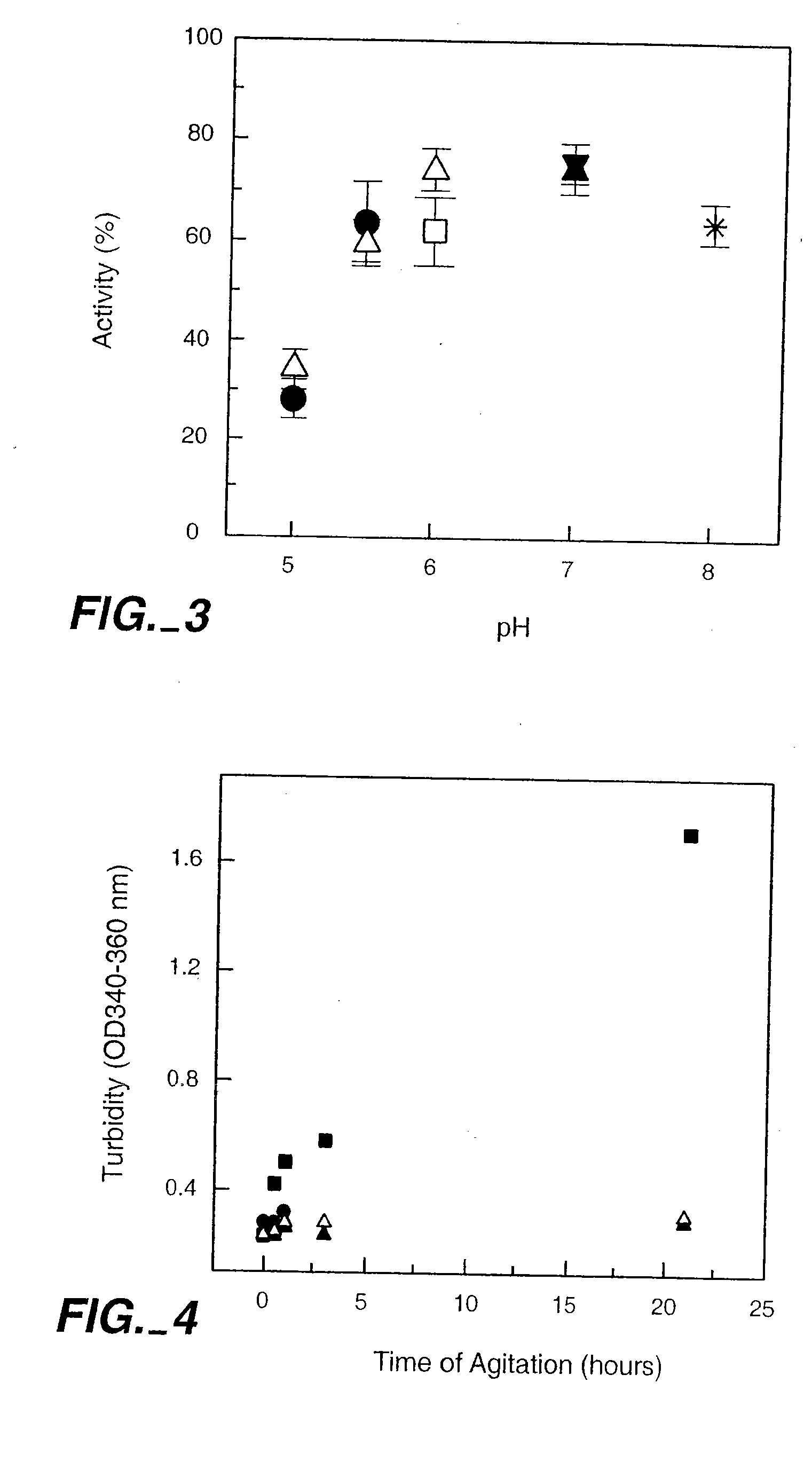
[0274]Agitation Study Aliquots of each formulation were placed in the glass vials. Vials were agitated horizontally on a Glas-Col Bench Top Shaker at room temperature. The shaker was set at 70 with an arm length of 30 cm (maximum). After agitation, samples were inspected and analyzed according to the following protocol.
[0275]Freeze-Thawing Study: Samples of E25 underwent three cycles of freeze-thaw. Each cycle consisted of freezing at −70° C. overnight and subsequently thawing at room temperature for about one hour. After each cycle, samples were inspected visually using a light box to assess the color and clarity of the liquid. Turbidity and soluble aggregates were measured following the protocol described below.
[...
example 3
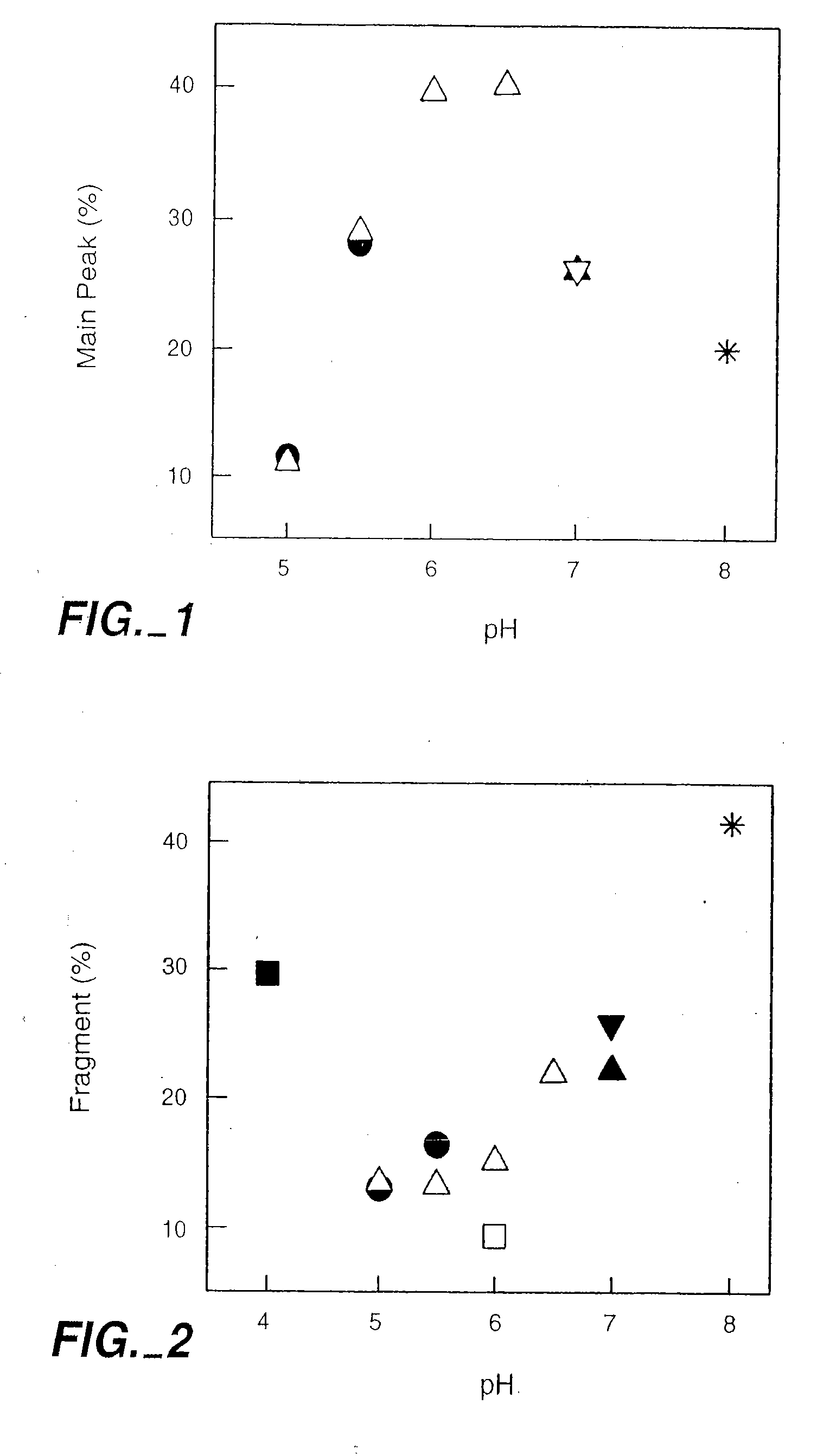
[0280]Samples of the anti-IgE monoclonal antibody (E26) liquid formulations were prepared in 20 mM buffers and then stored at 30° C. and 40° C. The stability of E26 was determined by chromatography and activity measurements. The size exclusion chromatography was used for determining the soluble aggregates, and the hydrophobic interaction chromatography of pepsin digested sample was used for measuring isomerization. The activity of sample was monitored by using an IgE receptor binding inhibition assay. As shown in FIGS. 1, 2 and 3, the degradation of E26 is highly dependent on pH of buffers. The E26 appears to be most stable around pH 6.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com