Interleukin-1 receptor antagonists, compositions, and methods of treatment
a technology of interleukin-1 and receptor, applied in the field of il1 receptor antagonists, can solve the problems of toxicity and secondary effects, limited treatment options for il-1-associated pathologies, and harmful actions of il-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of API-101 Peptide
[0194]One example of the efficacy of the compounds and methods of the present invention is represented by the results obtained with the identified peptide API-101 (SEQ ID NO 1: APRYTVELA).
[0195]All peptides described in the following examples have been synthesized according to the FMOC protocol of solid phase synthesis in an organic phase with protective groups. They have been purified with a yield of 70% with HPLC on a C18 column and eluted with an acetonitrile gradient of 10-60%. Their molecular weight have been verified by mass spectrometry. Of course as alluded above, when natural amino acids are used, they can be obtained by genetic engineering techniques as known in the art.
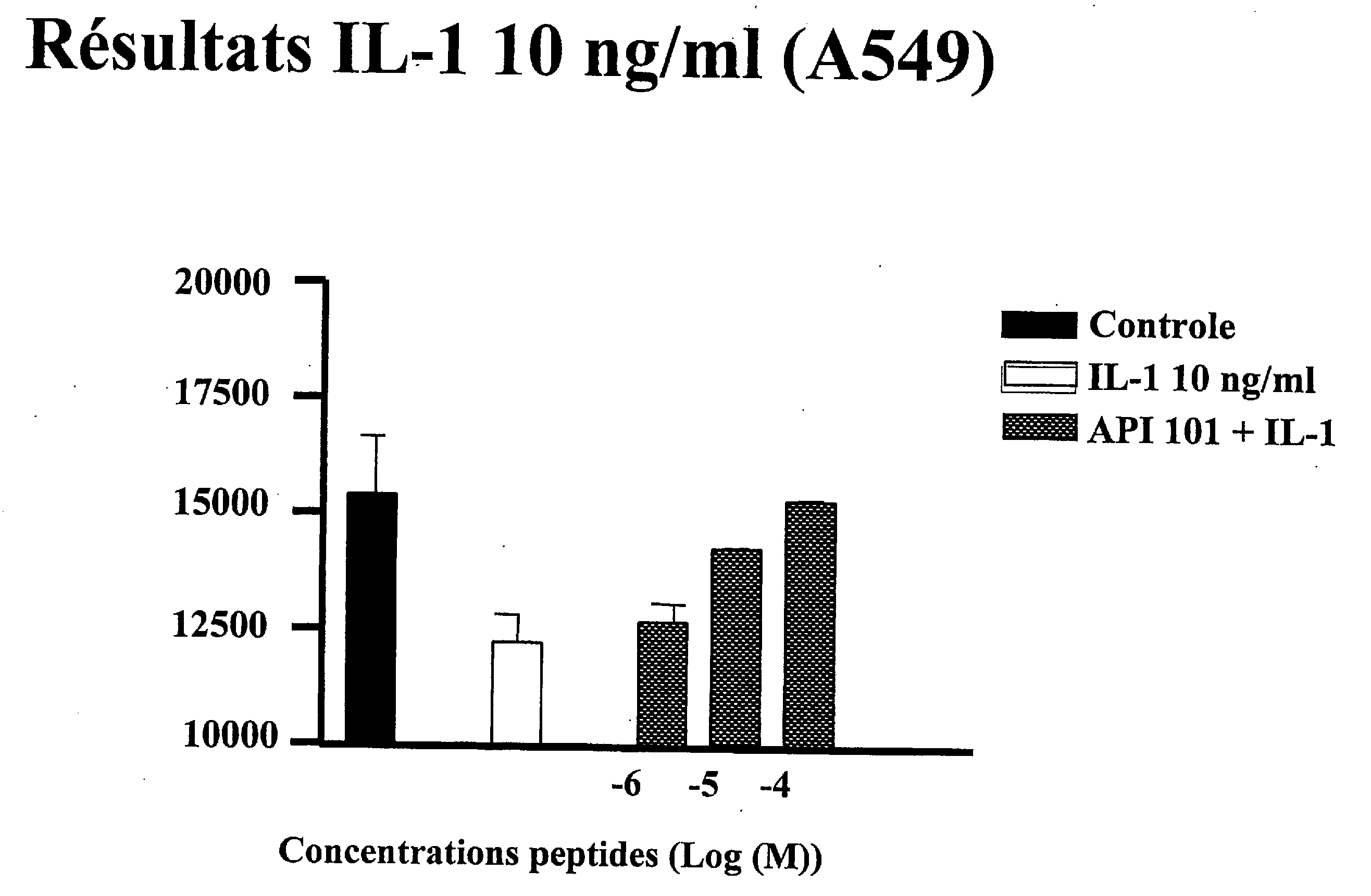
[0196]The proliferation effect of IL-1 was measured in A549 carcinoma cells in the presence of peptide API-101 (SEQ ID NO: 1) and of IL-1 (10 ng / ml) using the incorporation of tritiated thymidine method. A549 cells were pre-incubated (45 min.) with different concentrations of peptid...
example 2
Effect of Derivatives of API-101 Obtained by Alanine Scan
[0203]Having demonstrated a significant effect of the API-101 (SEQ ID NO: 1) antagonist, experiments were carried out to provide structure function relationship data for API-101 and derivatives, to identify the most important regions for activity. Alanine scan mutations were therefore performed on API-101 (SEQ ID NO: 1) (see FIG. 17 for the sequence of the peptides). Of course, other amino acids could have been used in the place of alanine to perform the scanning experiment.
In Vitro Characterization
[0204]A table summary of the results depending on the mutations performed is shown in FIG. 17.
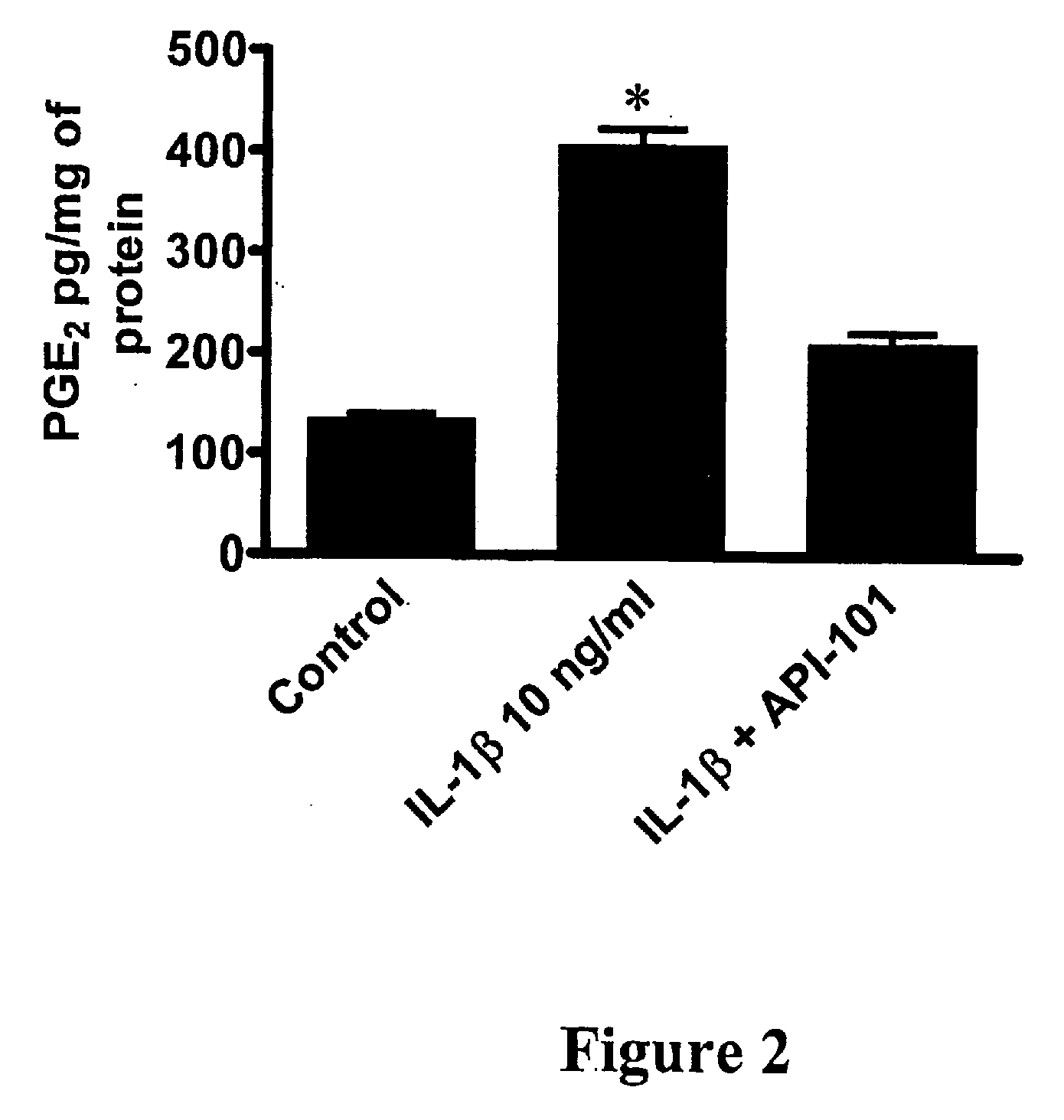
[0205]Efficiencies and inhibitory activities of the mutated peptides were determined by measuring the inhibition of IL-1-induced PGE2 synthesis (see experimental protocol above in Example 1). API-101.1 (SEQ ID NO: 2) only had a slightly improved efficacy in endothelial cells and in chondrocytes as compared to the parent peptide API-101 (SEQ...
example 3
Effect of Further Optimization of API-101 on the Improvement of its Activity
[0208]To further improve the activity and to validate the alanine scan conclusions obtained on the region in API-101 important for its activity, the amino acids from the N-terminal end of the peptide were gradually truncated. FIG. 7 shows the sequence of the new peptides as well as the general pattern of optimization employed for API-101.
In Vitro Characterization:
[0209]IL-1β induces proliferation of human fibroblasts cells. Truncated peptides were assayed for IL-1β induced WI-38 (human lung fibroblasts) proliferation with the tritiated thymidine uptake protocol (see protocol example 1).
[0210]Relative to API-101 (SEQ ID NO: 1) which abolished 65% of IL-1R induced proliferation; API-101.10 (SEQ ID NO: 10) and API-101.11 (SEQ ID NO: 11) abolished 100% of IL-1B-induced proliferation (FIG. 8).
[0211]Determination of IL-1-induced PGE2 synthesis was also performed on API-101 truncated derivatives. FIG. 18 shows a su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| protease resistance | aaaaa | aaaaa |
| serum stability | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com