Compositions and methods for treating or ameliorating mycobacterial infections
a technology for bacterial infections and compositions, applied in the direction of antibacterial agents, peptide/protein ingredients, endopeptidase, etc., can solve the problems of insufficient treatment to prevent the spread of the disease, the patient's behavior is often difficult to monitor, and the severity of complications and death is typically high, so as to achieve high activity and be recognized as useful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0198]The following specific examples are provided to better assist the reader in the various aspects of practicing the present invention. As these specific examples are merely illustrative, nothing in the following descriptions should be construed as limiting the invention in any way. Such limitations are, or course, defined solely by the accompanying claims.
example one
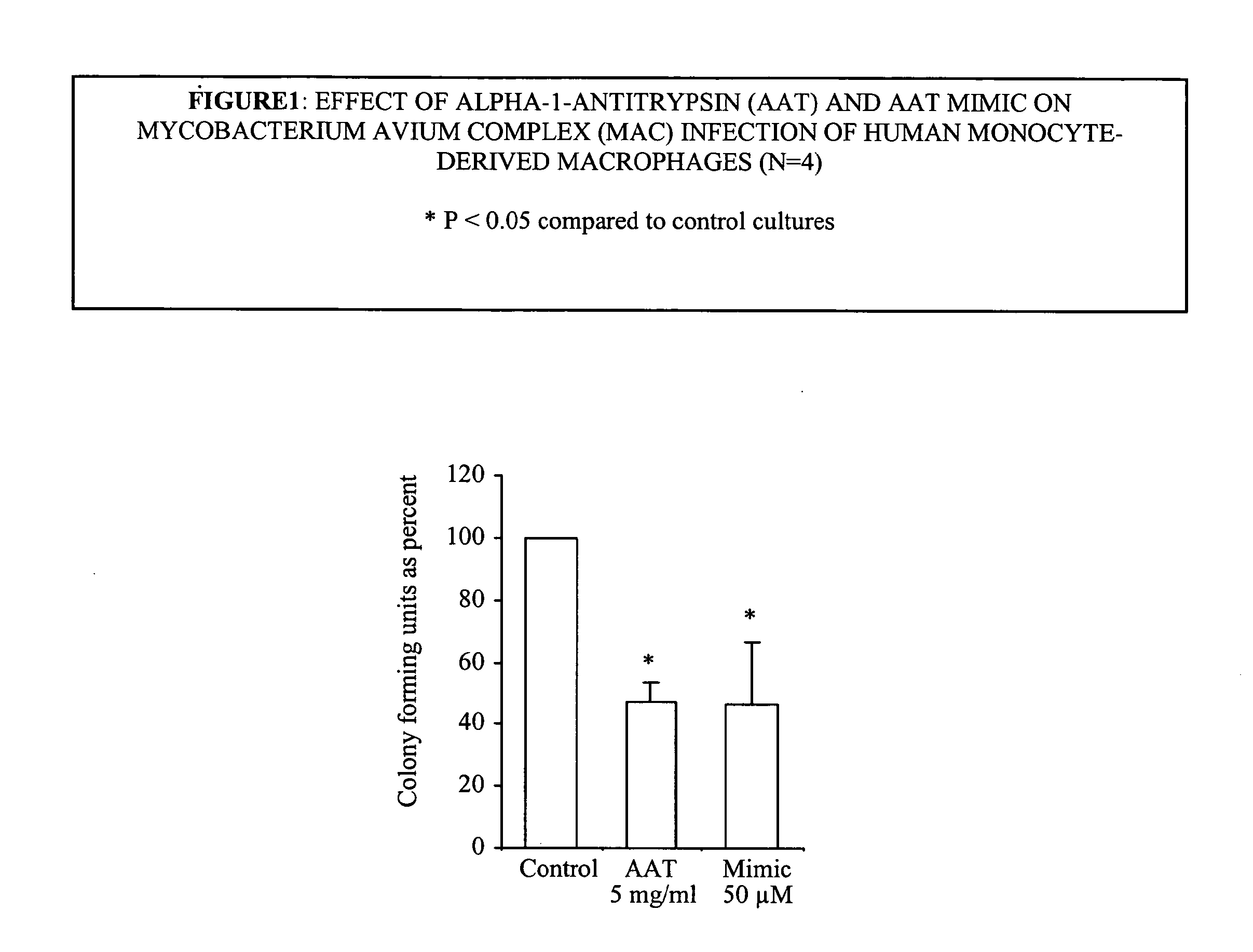
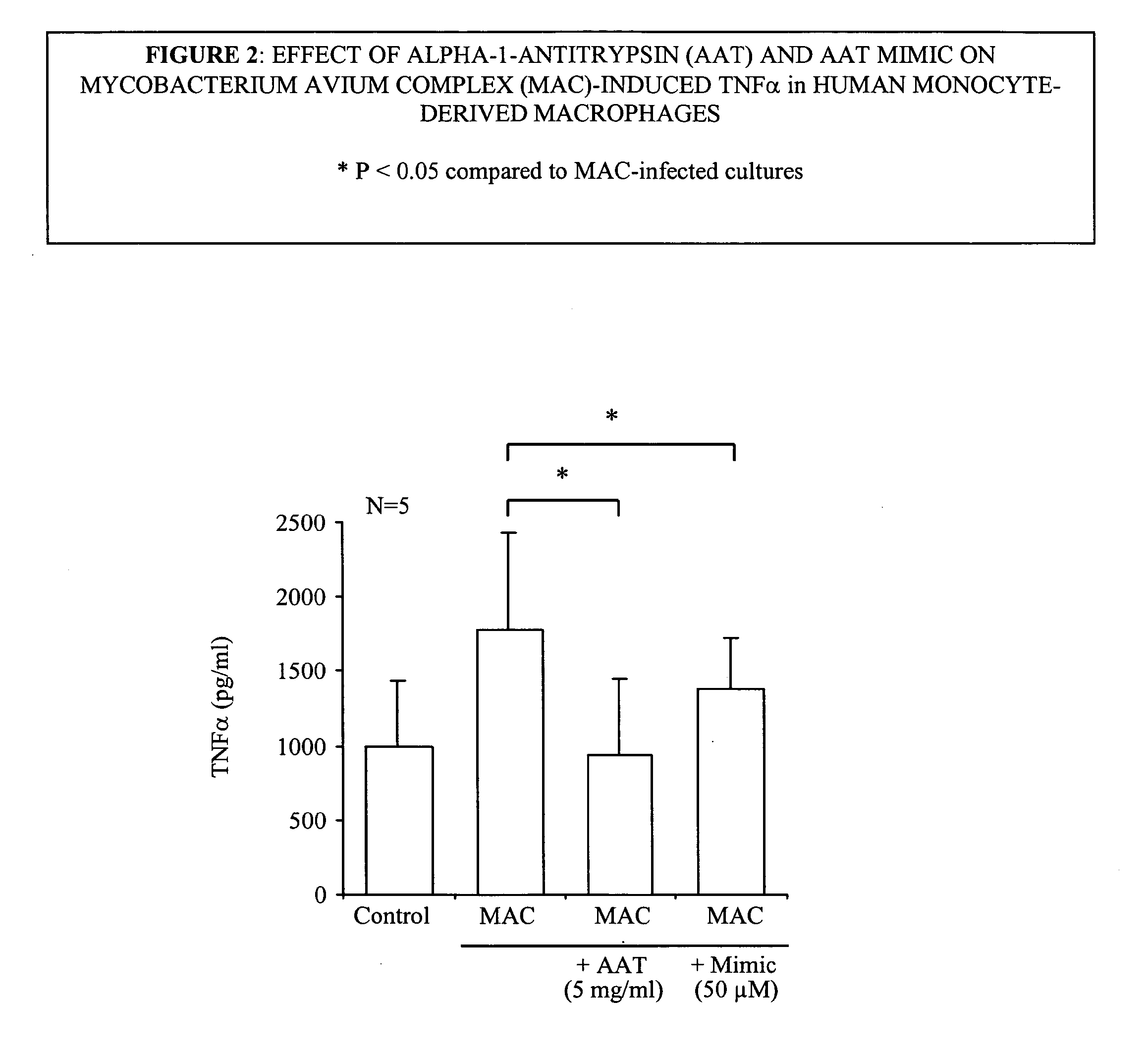
Effect of α1-Antitrypsin on Mycobacterium avium Complex (Mac) Infection of Human Monocyte-Derived Macrophages
[0199]1. TB or MAC organisms were suspended at a concentration of one Mcfarland standard. One McFarland is defined as a degree of turbidity of organisms suspended in liquid that matches that of a standard aliquot. A sample turbidity that is equivalent to that of the one McFarland standard represents about 10.sup.7 bacilli / ml. The optimal duration of a test culture is approximately 10-12 days of bacilli grown in Middlebrook 7H9 broth (=mycobacterium medium).
[0200]2. Infecting the cells. The cells infected were human monocyte-derived macrophages (MDM). MDM were isolated from human peripheral blood mononuclear cells (PBMC) that were obtained from heparinized blood from healthy volunteers by centrifuging the heparinized blood over a ficol-hypaque cushion. The isolated PBMC were aliquoted into polystyrene tissue culture plates and the monocytes are allowed to adhere .times.2 hrs (...
example two
Clinical Study in MAC Infection
[0217]The data described above in vitro using MAC have been supplemented with a clinical study. In this clinical investigation, AAT phenotypes (alternative forms of the AAT protein) were assessed in patients with documented lung infection with MAC and who had lung disease. These patients were compared to a control group consisting of patients with the lung disease bronchiectasis (in order to show that the presence of lung disease alone did not account for the presence of MAC infection).
BronchiectasisN = 134 subjectsMAC Infection (lungs)(lung disease)P-valueSex-Male8.97%23.21%Female91.3%76.79%Age (mean)64.5 yrs64.0 yrsATT phenotype (%0.006abnormal)-YES27.7% 5.3%NO72.3% 94.7%
[0218]Note in this table that that for the control (bronchiectasis) group, the proportion of patients with abnormal AAT molecules is 5.3%. This is in marked contrast to the case in the MAC.quadrature.infected group, where the proportion is 27.7%, a 5.2 fold increase. The MAC.quadratu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molar concentrations | aaaaa | aaaaa |
| molar concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com