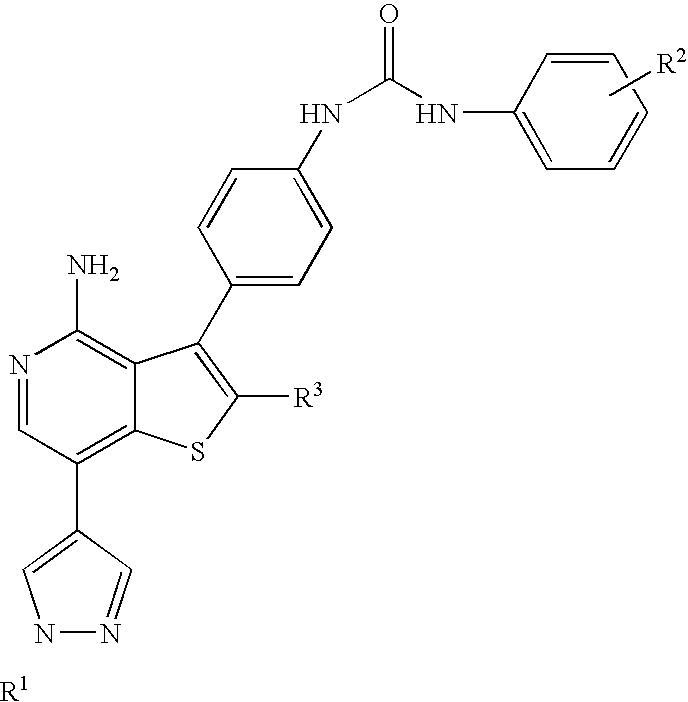
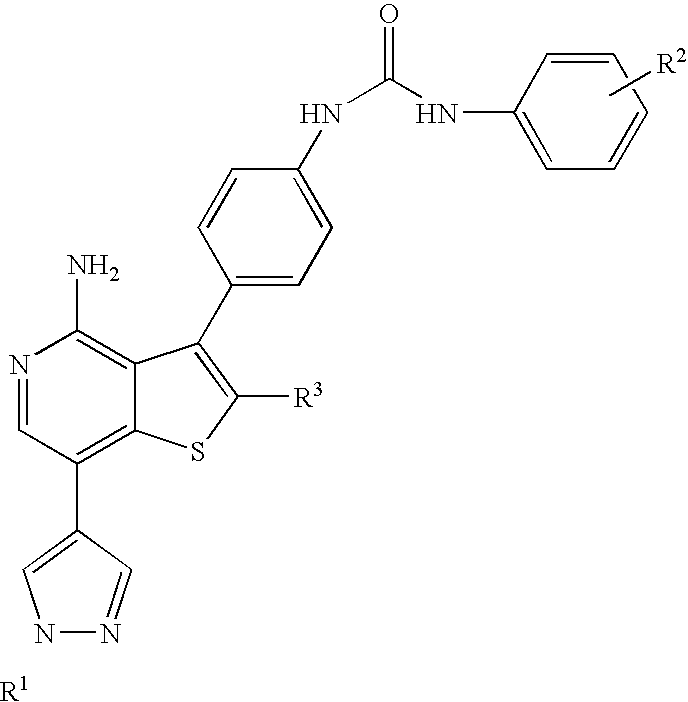
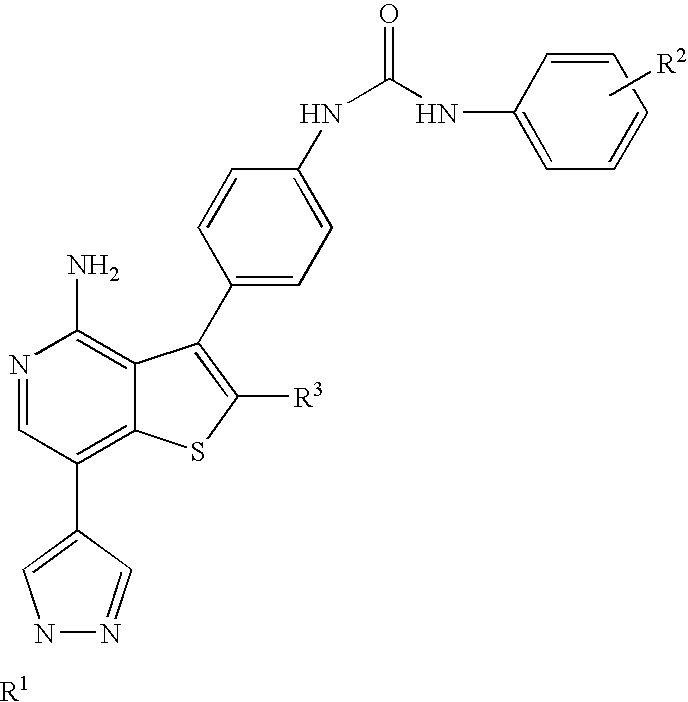
Kinase inhibitors with improved cyp safety profile
a technology of kinase inhibitors and safety profiles, applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of substantial increase or decrease in the blood and tissue concentration of a drug or metabolite, and the cyp enzyme is susceptible to inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
N-(4-{4-amino-7-[1-(2-hydroxyethyl)-1H-pyrazol-4-yl]thieno[3,2-c]pyridin-3-yl}phenyl)-N′-(3-fluorophenyl)urea
example 1a
3-(4-aminophenyl)-7-iodothieno[3,2-c]pyridin-4-amine
[0178]A suspension of 3-bromothieno[3,2-c]pyridin-4-amine (13.7 g, 59.7 mmol), tert-butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenylcarbamate (20 g, 62.7 mmol), tetrakis(triphenylphosphine)palladium(0) (2.5 g, 2.1 mmol) and Na2CO3 (13.3 g, 125 mmol) in tetrahydrofuran (150 mL), methanol (40 mL) and water (80 mL) was degassed, then stirred at reflux overnight. The reaction mixture was cooled to room temperature, then partitioned between ethyl acetate and water. The aqueous layer was extracted with additional ethyl acetate and the combined organics were dried (using MgSO4), filtered and the filtrate was concentrated. The residue was purified via silica gel chromatography eluting with 50 to 70% ethyl acetate-hexanes to give crude tert-butyl 4-(4-aminothieno[3,2-c]pyridin-3-yl)phenylcarbamate. A solution of the crude product (59.7 mmol based on 100% yield) in N,N-dimethylformamide (80 mL) was treated with N-iodosuccinimide (...
example 1b
2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)ethanol
[0179]4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (9.66 g, 49.8 mmol), 1,3-dioxolan-2-one (21 g, 238 mmol) and cesium carbonate (16 g, 49.1 mmol) were combined in a 100 mL round bottom flask. The reaction was warmed from room temperature to 100° C. in an oil bath, by which time the carbonate had melted and served as the solvent for the reaction, which remained a slurry. After heating for 3.5 hours, the reaction was cooled to room temperature and diluted with ethyl acetate, then filtered through Celite washing repeatedly with ethyl acetate. The filtrate was concentrated, then purified by chromatography on an Analogix® Intelliflash™ purification system using a SF60-200 g column at a flow rate of 80 mL / min, eluting as follows: 5 minutes at 20% ethyl acetate / hexanes, then ramped from 40% to 90% ethyl acetate / hexanes over 35 minutes, then 100% ethyl acetate for another 20 minutes, to provide the tit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time- | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com