Constitutive expression of costimulatory ligands on adoptively transferred t lymphocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
T Cells Co-expressing CD80 and 4-1BBL Elicit Robust Proliferative Responses after Cyclic Stimulations through their Endogenous T Cell Receptor or through a Chimeric Antigen Receptor without Antigen Presenting Cell (APC)-provided Costimulation
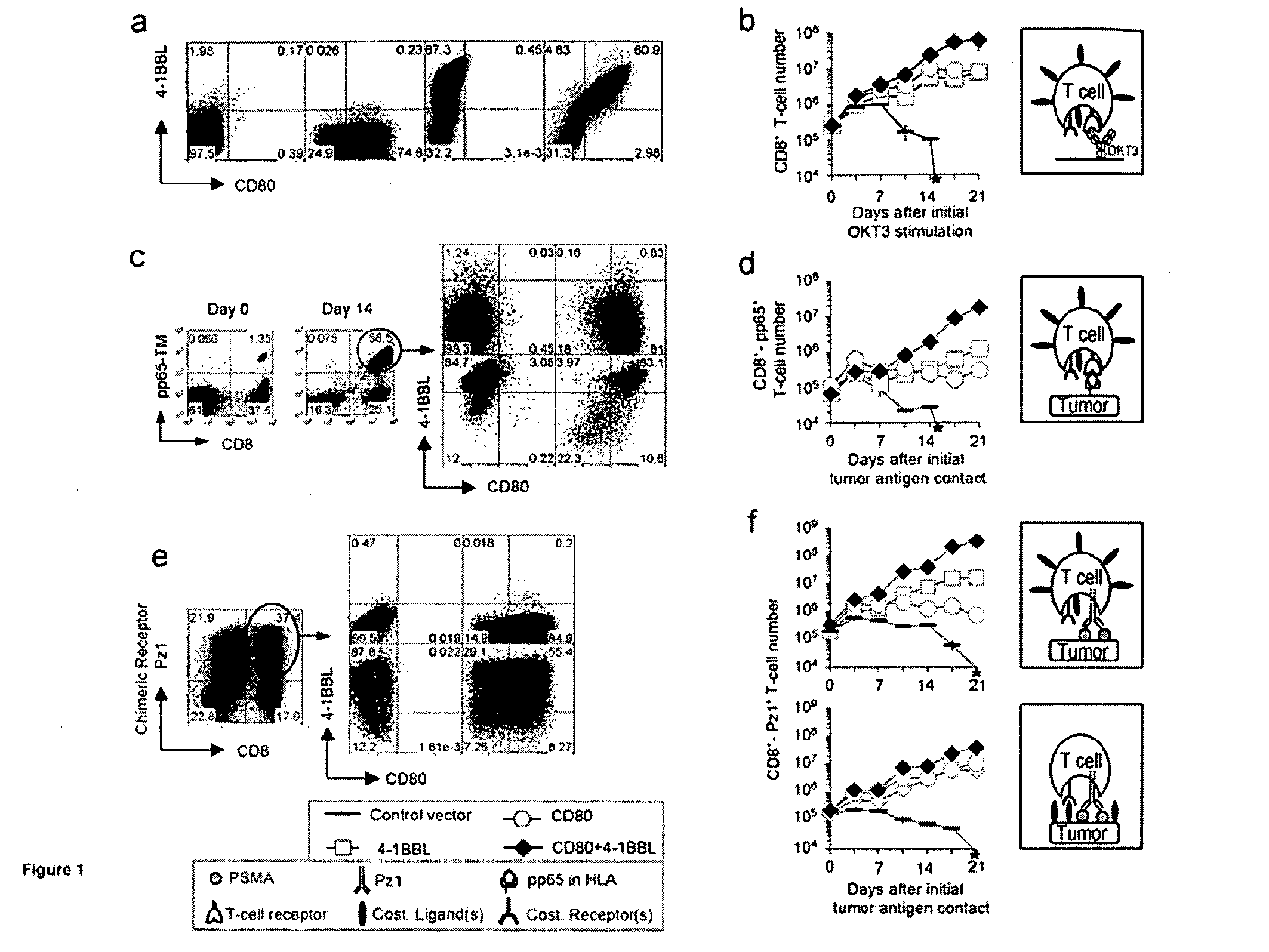
[0141]To assess whether constitutive expression of costimulatory ligands in T cells could substitute for APC-mediated costimulation, the T cell responses of human primary T cells were first investigated in three experimental systems. Using anti-CD3 (OKT3)-mediated T cell activation, the expansion of peripheral blood T lymphocytes transduced with CD80 and 4-1BBL was quantified (FIG. 1a), which were compared to T cells transduced with either ligand alone or none. Recurrent T cell receptor (TCR)-stimulation alone in the absence of costimulatory ligands failed to expand T cells and rapidly induced a decline in T cell number following the first restimulation (FIG. 1b). In sharp contrast, OKT3-stimulated CD80+4-1BBL+ T cells triggered a mean 237-fold ...
example 2
T Cells Co-expressing CD80 and 4-1BBL Eradicate Established, Systemic Tumors.
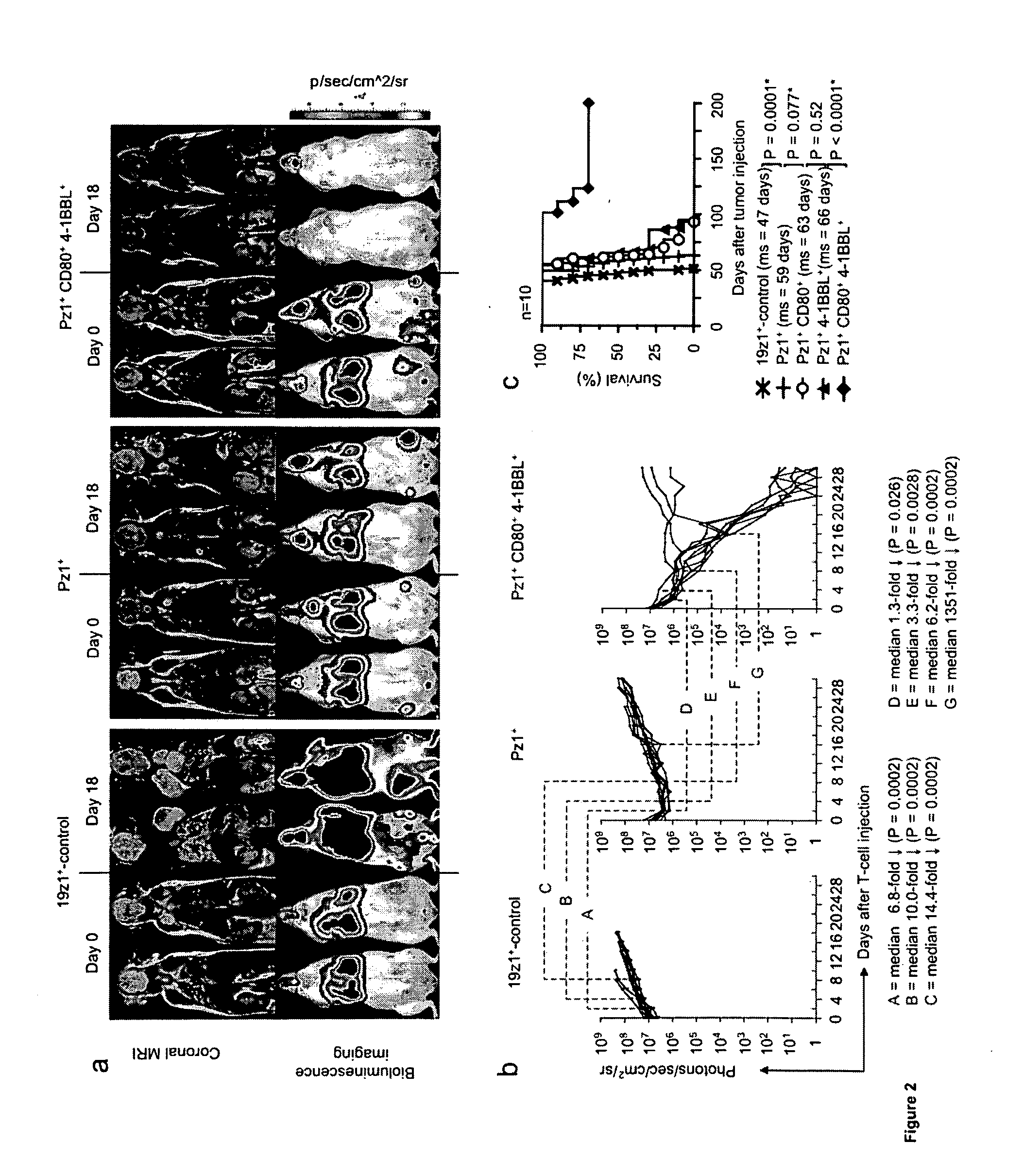
[0144]To investigate the potency of our CD80+4-1BBL+ T cells in vivo, a model of multifocal, established prostate cancer utilizing PSMA−PC-3 tumor cells (Gong et al., Neoplasia 1:123-7, 1999, which is hereby incorporated by reference in its entirety) was developed. Using dual-modality bioluminescence and magnetic resonance imaging, tumors were visualized four weeks after intravenous inoculation, prior to initiating adoptive T cell therapy. The lungs, cervical lymph nodes, bone marrow, and liver were identified as the main sites of disease (FIG. 2a). In this model, animals were treated four weeks after tumor inoculation with a single intravenous infusion of 8×106 PSMA-targeted T cells, expressing either CD80, 4-1BBL, both, or neither.
[0145]In control mice treated with 8×106 CD19-targeted T cells, which, like untransduced T cells, fail to lyse PSMA+ tumor targets in vitro tumor burden steadily progressed unti...
example 3
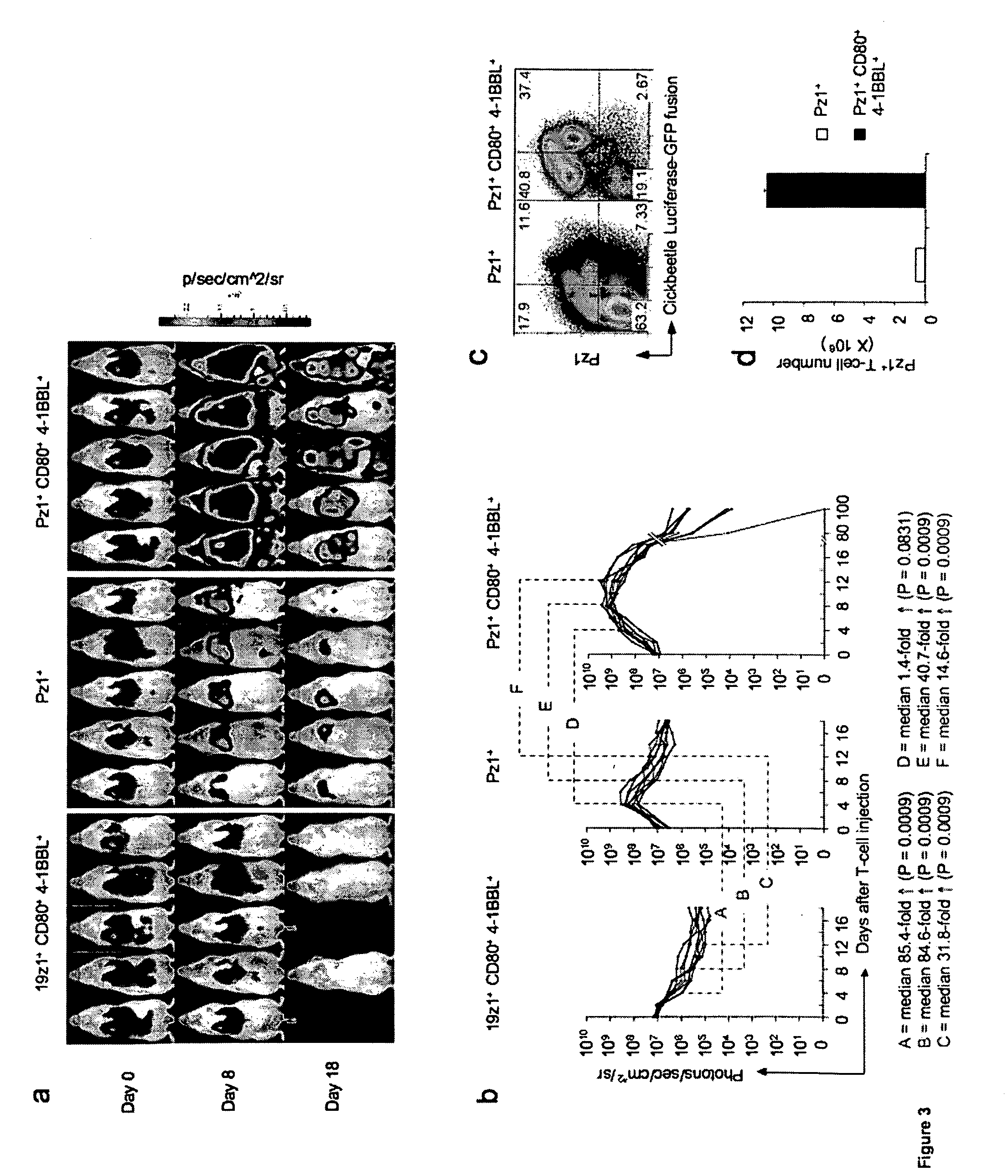
In vivo T Cell Expansion is Robust and Antigen-specific.
[0146]To track and quantify in vivo T-cell migration and accumulation in relation to tumor localization and tumor burden, adoptively transferred T cells were additionally marked with Click Beetle Red-luciferase (Ponomarev et al., Eur J Nucl Med Mol Imaging. 2004 May; 31(5):740-51) (CBR-luc, FIG. 3a). Serial imaging of mice treated with Pz1+ T cells showed a progressive increase in signal that reached a peak four days after T cell injection (FIG. 3b). A low-level signal remained detectable up to day 18. In the case of Pz1+CD80+4-1BBL+ T cells, peak signal was detected on day 8 (41-fold higher nadir photon count than Pz1+ T cells, p=0.0009), which was followed by a gradual signal decline, although bioluminescence could still be detected until day 100 in some animals (FIG. 3b). Importantly, the effect of CD80 and 4-1BBL co-expression was abrogated in 19z1+ T lymphocytes (FIG. 3b), consistent with the need for antigen stimulation f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com