Compositions and method for storage of nucleic acid from bodily fluids
a nucleic acid and body fluid technology, applied in the field of compositions and methods for the isolation and storage of nucleic acids from bodily fluids, can solve the problems of blood as a source, multiple steps that can be time-consuming and expensive, and the typical content of dna source materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Protocol for Obtaining Saliva Samples from Subjects Capable of Following Instructions
[0055]The subject is instructed to wait for a period of 30-60 minutes before last eating. The subject will brush his teeth without using toothpaste, if possible. The subject will rinse his / her mouth with 50 ml of cool or tepid water. The subject will be requested to wait for 2 minutes to allow the mouth to clear of water, then spit saliva into the special collection tube until the level of saliva reaches the 1 ml mark. Waiting after last eating and rinsing the mouth is desirable but not essential. Collection of saliva may take several minutes. If the subject finds that he / she is unable to deliver sufficient saliva, he / she will be given a few grains of table sugar to chew, and told not to be concerned if some of the sugar is spit into the tube.
[0056]Where two or more samples are to be taken from a donor for purposes of comparing two compositions, the donor is asked to deliver small amounts of saliva ...
example 2
Stability of DNA in Saliva and PCR Amplification of Saliva-Derived DNA with a Heating Step
Method
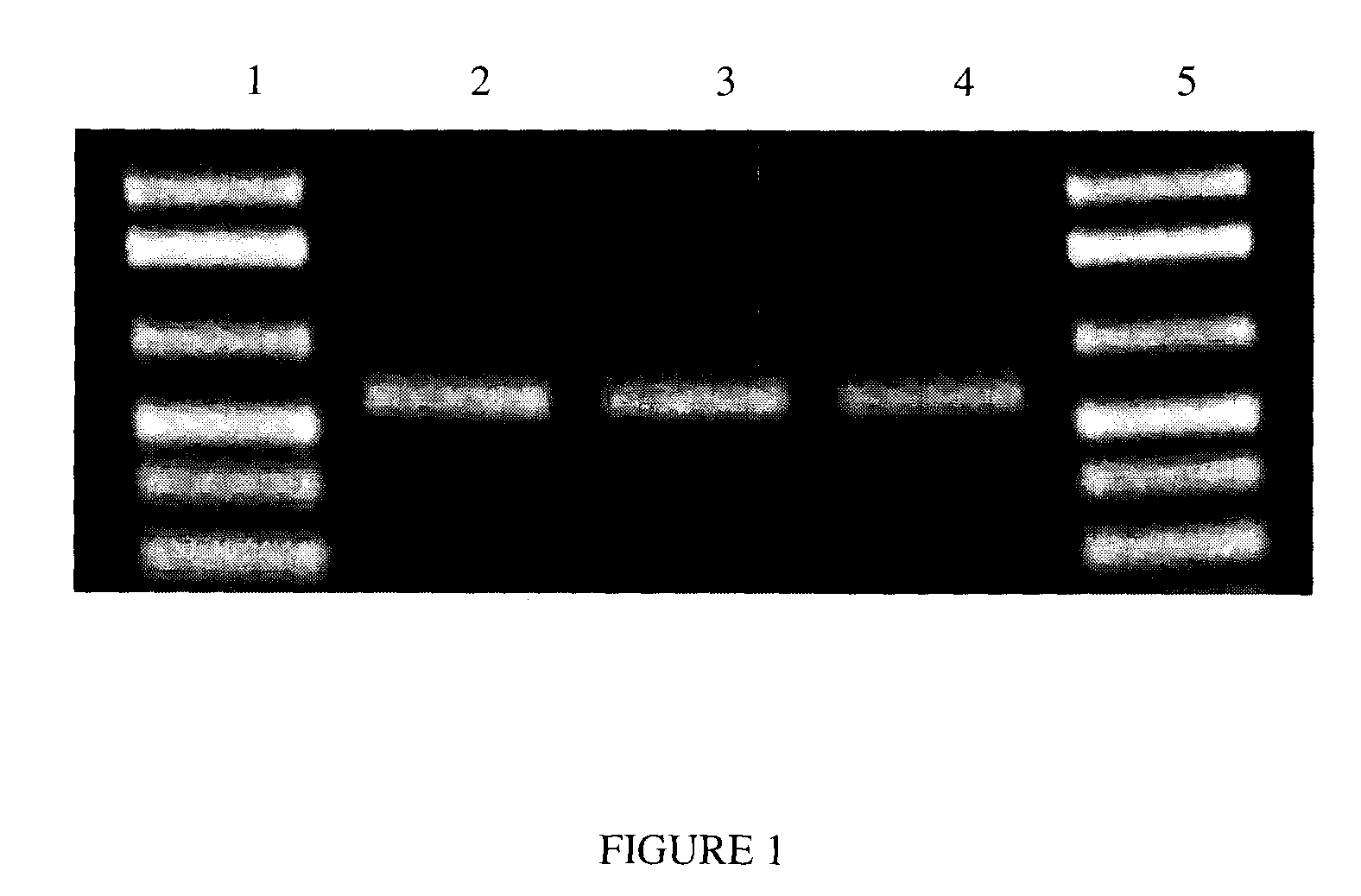
[0058]Saliva was collected from a single donor, using the protocol described in Example 1, and individual samples of the saliva were mixed 1:1 with a solution consisting of 20 mM CDTA; 400 mM NaOAc (sodium acetate); 200 mM Tris-HCl, 0.05% SDS (sodium dodecyl sulfate); pH adjusted to 8.0. 20 μL of Proteinase K (1 mg / mL concentration) was then added to this mixture (10 μg / mL final concentration). The samples were vortexed, and then allowed to sit at room temperature (approximately 24° C.). At Day 1, Day 14, and Day 365, 2 μl of the saliva / solution sample was added directly to a standard 50 μl PCR reaction following incubation for 1 hour at 60° C.
PCR Conditions
[0059]PCR reactions were performed in an Eppendorf Mastercycler™ gradient PCR machine. The total reaction volume was 50 μL. Each reaction contained Invitrogen PCR Mastermix plus Tris-HCl (pH 8.0, 10 mM final concentration), MgCl2 (2 mM...
example 3
Stability of DNA in Saliva and PCR Amplification of Saliva-Derived DNA
Method
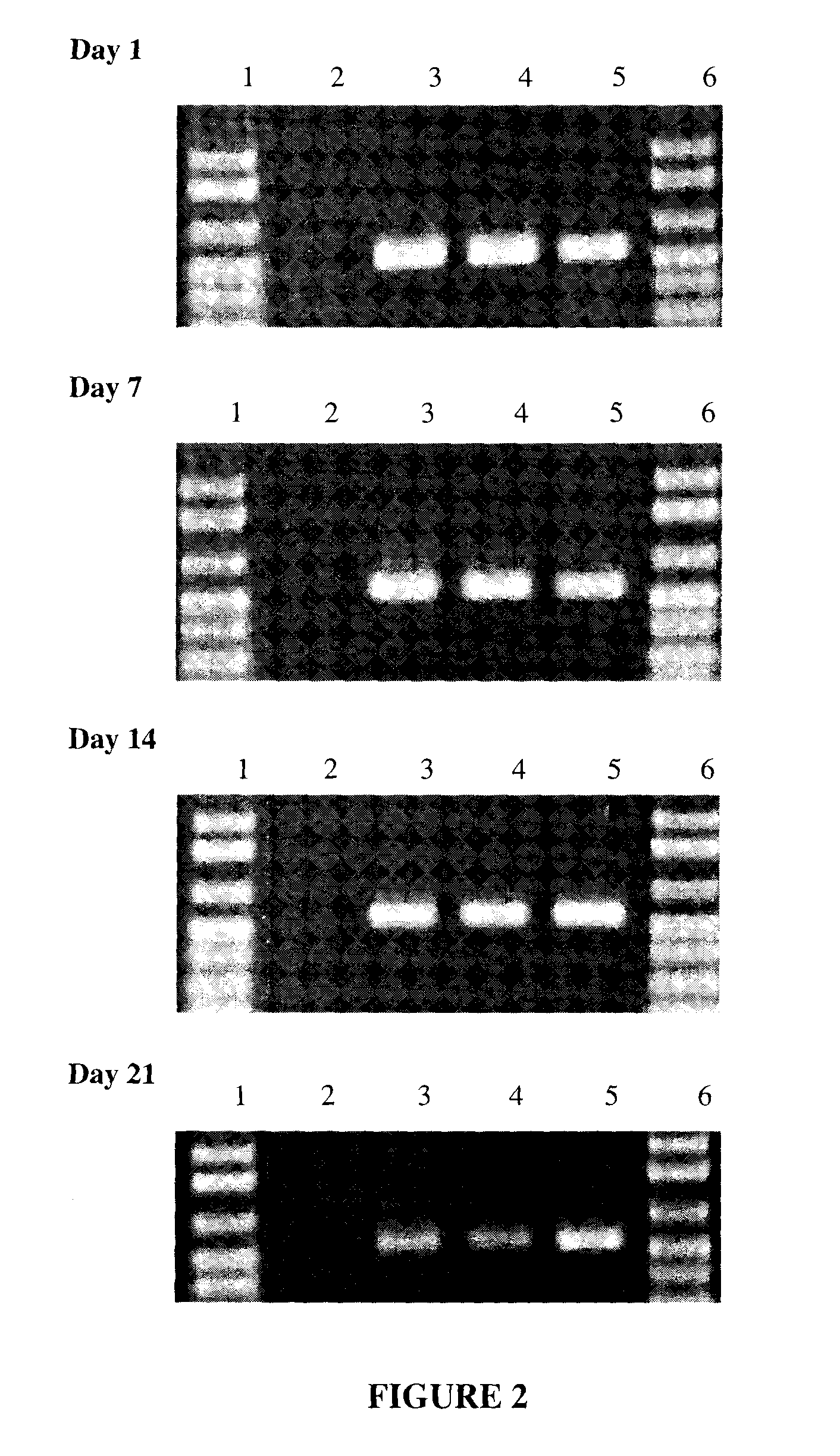
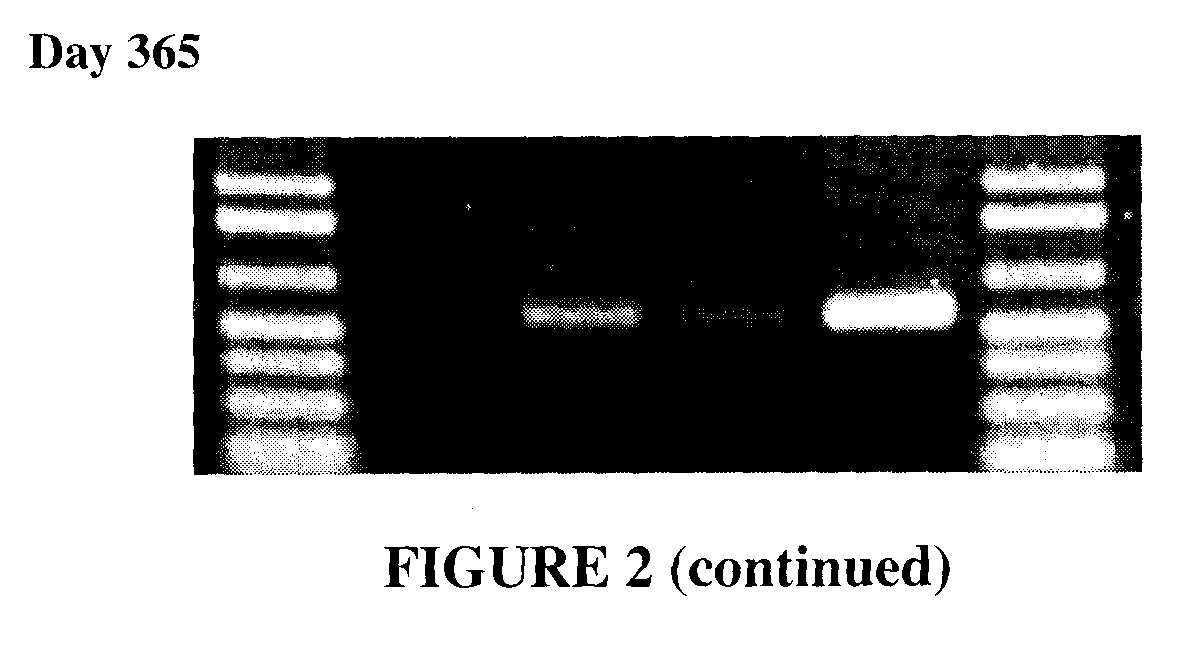
[0063]Saliva was collected from a single donor, using the protocol described in Example 1, and individual samples of the saliva were mixed 1:1 with each of the following solutions:[0064]water[0065]100 mM NaOH (sodium hydroxide)[0066]100 mM KOH (potassium hydroxide)[0067]200 mM Na2CO3 (sodium carbonate)
[0068]The samples were vortexed and allowed to sit at room temperature (approximately 24° C.). At Day 1, Day 7, Day 14, Day 21, and Day 365, 2 μl of the saliva / solution sample was added directly to a standard 50 μl PCR reaction.
PCR Conditions
[0069]PCR reaction conditions were the same as in Example 2. Thermocycling conditions were the same as in Example 2.
[0070]Agarose gel electrophoresis was performed as described in Example 2.
SUMMARY
[0071]As can been seen in FIG. 2, DNA from saliva was stable and usable for PCR when mixed 1:1 with 100 mM NaOH, 100 mM KOH and 200 mM Na2CO3, and store...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com